Nature Communications article: An ATPase mediates autophagosome formation

First author
Autophagy is a cellular degradation process that entails sequestration of cytoplasmic material by double-membrane autophagosomes. When an autophagosome fuses with a lysosome, the degradative enzymes of the lysosome will degrade the sequestered material. The autophagosome is formed from a structure called the phagophore, but it has remained unclear where the phagophore comes from and how it is shaped into an autophagosome.
Subdomains of the endoplasmic reticulum (ER) known as omegasomes (because of their omega shape in fluorescence microscopy), which are characterized by the protein DFCP1, have been proposed to promote shaping of phagophores,
Now, researcher Viola Nähse and her co-workers in Harald Stenmark´s group, in collaboration with associate professor Kay Schink, have shown that DFCP1 is an ATPase that constricts omegasomes and in this way promotes autophagosome formation. Its activity is particularly important for the formation of large autophagosomes involved in selective autophagy of large cytoplasmic objects such as damaged mitochondria and protein aggregates.
Selective autophagy plays crucial roles in preventing both cancer development and neurodegenerative diseases, and the current findings are therefore particularly interesting in this context.
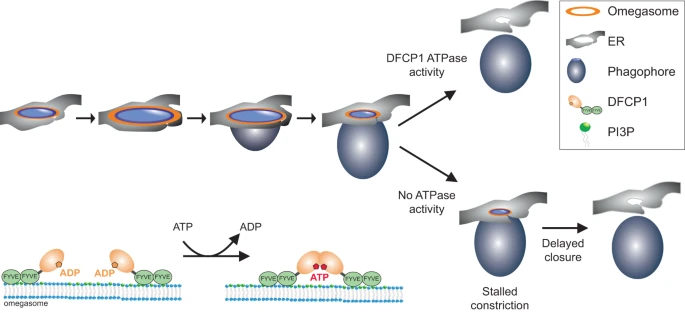
Upon induction of autophagy, PtdIns3P is generated at specialized subregions of the ER, at omegasomes. A DFCP1 spot is formed de novo, to which p62 and LC3B are recruited a few seconds later. The spot grows and forms a ring-like structure. Omegasomes first expand, and the phagophore, likely originating from a cytoplasmic seed vesicle, extrudes out of the ring and forms a pocket. Then, the omegasome ring constricts and finally, the phagophore is closed and becomes an autophagosome. DFCP1 localizes to the omegasome throughout the whole process of autophagosome formation. ATPase activity of DFCP1 is required during the constriction of the omegasome. In cells expressing WT DFCP1, the omegasome rapidly constricts. In contrast, in cells expressing ATPase mutants of DFCP1, the constriction stalls, delaying the closure of the omegasome. On a molecular scale, ATP binding and hydrolysis controls dimerization state of DFCP1 which could provide the mechanochemical energy to drive this constriction.
(Fig. 6 from the Nature Communications article)
Links:
The Nature Communications article:
ATPase activity of DFCP1 controls selective autophagy.
Nähse V, Raiborg C, Tan KW, Mørk S, Torgersen ML, Wenzel EM, Nager M, Salo VT, Johansen T, Ikonen E, Schink KO, Stenmark H.
Nat Commun. 2023 Jul 8;14(1):4051. doi: 10.1038/s41467-023-39641-9.
PMID: 37422481
Viola Nähse, first author
The home page of Harald Stenmark's group Cellular membrane dynamics
Department of Molecular Cell Biology
