The Innovative science 2019 award-winning LUMIBLAST concept: Fighting brain cancer through photodynamic therapy, using light from within

Recently Oslo University Hospital was awarded by the European Commission the first prize for best innovative science 2019, for the inception of the idea of the ongoing Future and Emerging Technologies Open project: LUMIBLAST, coordinated by Kristian Berg and Theodossis Theodossiou.
The competition held by the Innovation Radar of the EU. After a pre-selection of 9 projects per category amongst a large number of potential candidates, there were two final competition phases: first public voting in which OUS came the third finalist and made it through to the next phase, which involved a three-minute pitch from each candidate, in front of a jury of entrepreneurs and investors. The pitching competition was carried out at 15-17.00 on the 25th September (view video from pitching contest - you may skip to 1h.14m.50sec), while after an overnight deliberation the prizes where revealed on the 26th of September, at the closing ceremony of the innovation hub.
Crucial for the overall competition was the intervention of our institute leader Kjetil Tasken to publicize the voting to the whole of OUS through an internal news release and alert people to the competition, while several of our artistically gifted colleagues in the Institute for Cancer Research (Laura Marian Valencia Persqueira, Simona Migliano, Anna Barkovskaya and Caroline Dillard-Eple) contributed dedicated artwork, specifically for the needs of the pitch .

|

|
About LUMIBLAST
It is a fact that cancer is one of the two major causes of death second only to heart disease. But what is the worst form of cancer that someone can have?
That would have to be an incurable deep lying cancer, like the brain cancer glioblastoma multiforme (GBM). It is an aggressive form of brain cancer, rapidly disseminating within the patient’s brain, rare, yet deadly. This is the problem we chose to initially address with LUMIBLAST.
Currently there are 3 treatment options for GBM. Surgery, chemotherapy with temozolamide and radiotherapy. However, the combined application of all three, leads to a median survival of 14 months as opposed to 3 months without any treatment.
On the other hand, there are some photoreactive drugs that can so specifically accumulate in the tumours, so when you shine blue light, the cancerous tissues glow red.
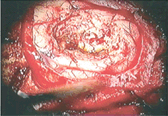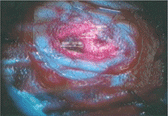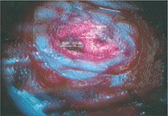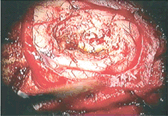
This has been used to precisely guide the surgical resection during open skull GBM operations. Furthermore, when irradiated with more intense light, these substances can be used for a cancer-treatment called photodynamic therapy, destroying the cancer around them through the production of reactive oxygen species, and specifically singlet oxygen.
There is a limitation though: Light cannot penetrate deep in tissue, let alone the skull...
But what if we used a chemiluminescent substance e.g. luminol (used in forensics to detect blood) as an internal light source as opposed to an external one, e.g. laser or lamp as conventionally used in PDT?
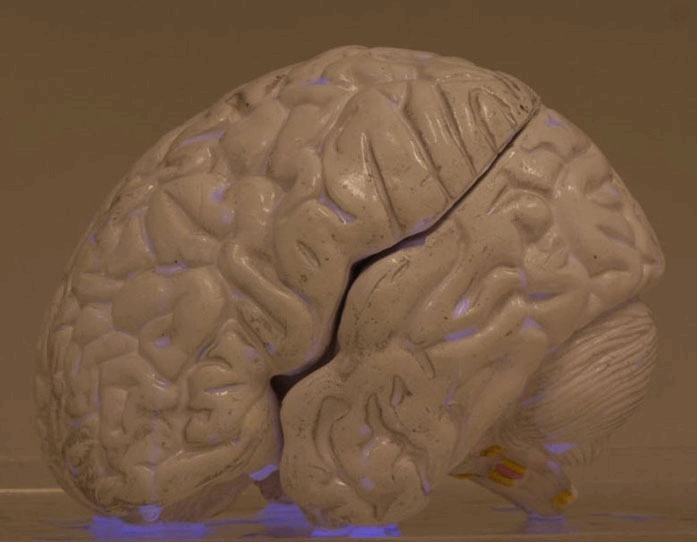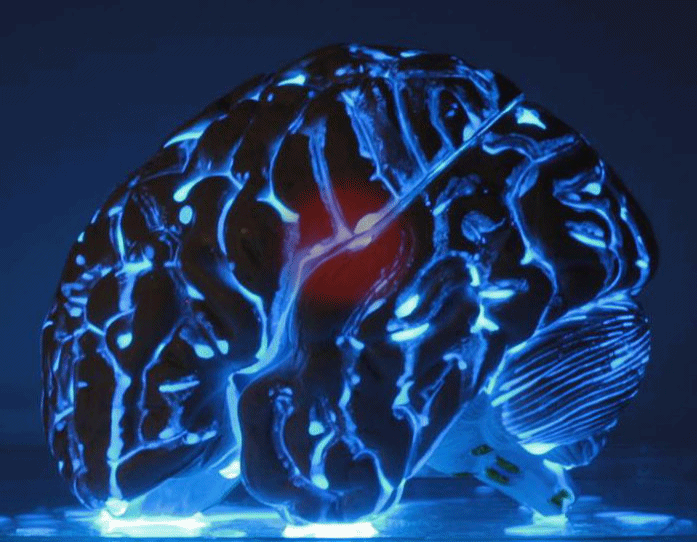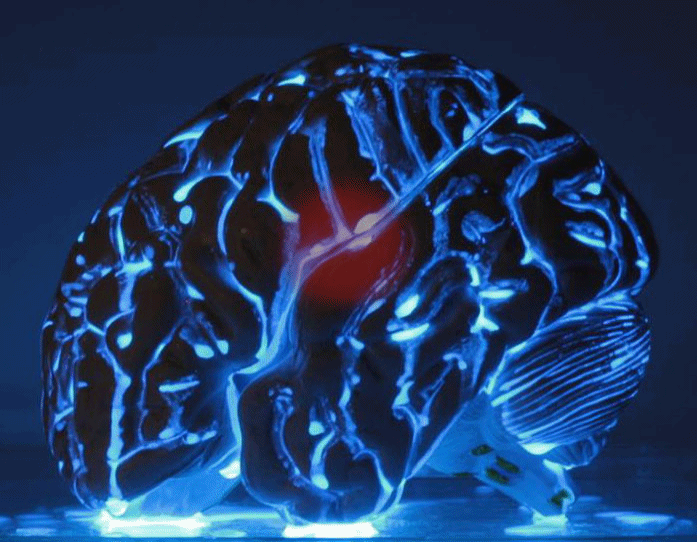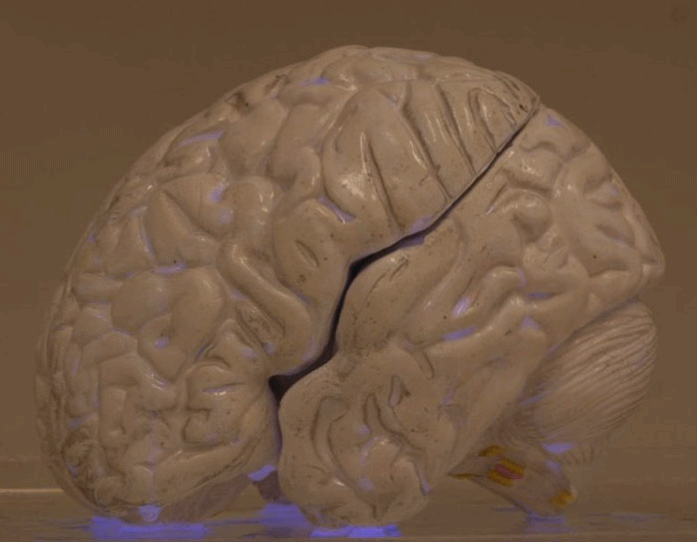
This is the LUMIBLAST concept: with the combined administration of 2 individually non-toxic chemicals, each cancer cell becomes a miniature lamp and since the photoactive drug is specifically accumulated in the cancer → the cancer cells “commit suicide”, in a modified version of chemiluminescence PDT.
This all happens within the cell mitochondria (the cell power factory). The light is self-sustained as it is powered by the mitochondria, which is also where the PS is located... So key to the project was the synthesis of proprietary luminol molecules that end up in the mitochondria to be close to the PS.
We started the project in 2016. This was after a one stage application receiving a score of 95% and ranking 6thamongst the 11 successful proposals out of 821 applications (success rate 1.4%). In the current stage, 3 years into the project, 80% of the chemical synthesis has been completed while the successful chemicals have been identified through validation in cells. Next, the main LUMIBLAST patent will be published in December 2019 while the initial proof-of-principal in animal models is expected within 2020. When the FET project is concluded we will solicit investors for advanced preclinical and clinical studies. The holy grail is to make LUMIBLAST an effective clinical reality.
For that purpose, and whilst LUMIBLAST is modelled on GBM, it is not the only grim cancer case. We have already made a draft survey regarding the clinical/market prospects of LUMIBLAST and have singled 3 difficult to cure indications, each with low survival prognosis, to which LUMIBLAST can be applicable, i.e. pancreatic cancer, cholangiocarcinoma (bile duct cancer) and mesothelioma.
The Lumiblast consortium comprises one hospital (coordinator Oslo University Hospital) three academic institutions (National and Kapodestrian University of Athens, Polytechnic University of Valencia and Oslo University) and one SME (Knight Scientific Ltd).

Links:
From the Europaen Commission home pages:
Innovators competing in the 'Innovative Science' category of the Innovation Radar Prize 2019
Winner of the 2019 Innovation Radar Prize announced
Kristian Berg's group: Photochemical internalisation (PCI)
Theodossis A. Theodissiou's project group: Protonics
