IGL CAR
IGL CAR (S. Wälchli):
Chimeric Antigen Receptor (CAR) therapy is a promising treatment for cancer. The FDA and the EU approved a commercialized treatment of B-cell malignancies targeting CD19 antigen. However, targeting CD19 can result in B-cell aplasia, which leads to increased susceptibility to infections that can be fatal. Since B cell receptors (BCR) have a bi-allelic restriction of Immunoglobulin (Ig) light chain expression, Ig-Kappa (Igκ) or Ig-Lambda (Igλ), targeting one allele will protect healthy cells from the other. To date only two anti-IGK have been reported (Vera et al. 2006 and Köksal et al. 2019). We have identified the sequence encoding the antigen-binding parts of an anti-Igλ antibody from our collection and designed a second-generation CAR construct (IGL CAR). IGL CAR redirected peripheral blood T cells were tested in various in vitro assays with target cells and compared with the clinical CD19 CAR (clone fmc63). IGL CAR T cells demonstrated specific killing and cytokine release against Igλ+ cells, such as MAVER-1, while no response was detected against Igκ+ cell lines such as BL-41. We are presently developing a combinatorial construct to avoid serum IgL to interfere with IGLCAR-Tc.
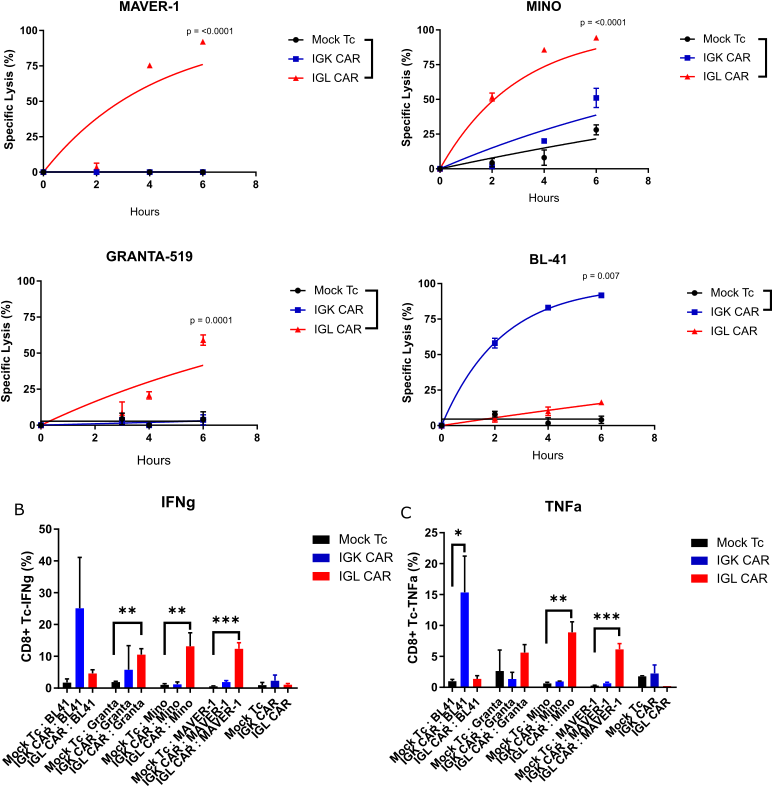
IGK CAR
IGK CAR (S. Wälchli/B. Caulier):
Chimeric antigen receptor (CAR) based immunotherapy is coming under the spotlight in the cancer treatment. This is mainly due to the success of CAR T cells targeting B-lymphocyte antigen CD19, which has led to astonishing results in clinical trials. Considering that all B cells express CD19 antigen, CAR-T cells eliminate all B cells, including non-malignant B cells. Therefore, the patients suffer from impaired humoral immune response, specifically B-cell aplasia and hypogammaglobulinemia, which might increase susceptibility to severe infections. Another problem is related to the target itself. Accumulation of data demonstrates the possibility of immune escape by down regulation of CD19 or alternative splicing variant which becomes resistant to standard CD19 CAR. There is therefore a need for alternative targets. Taking into account that most B-cell lymphomas and chronic lymphocytic leukemia cells have a clonally restricted expression of Immunoglobulin (Ig) light chains, either Ig-kappa or Ig-lambda, Ig-kappa+ tumor cells can be targeted while sparing normal Ig-lambda+ B-cells. Hence, Ig-kappa CAR T cells could provide lower on-target toxicity than CD19 CAR T cells and would be expected to improve the life quality of the patients.
You can learn more here.
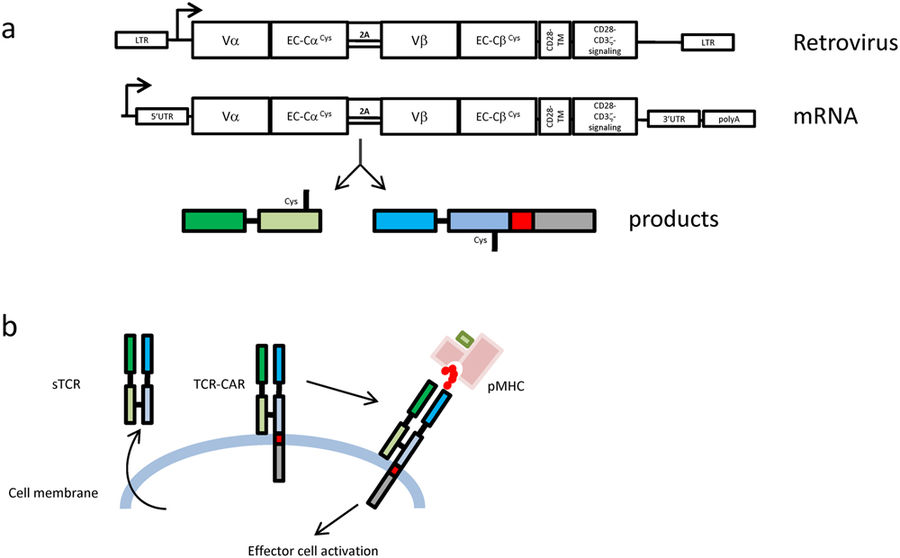
TCR CAR
TCR CAR (S. Wälchli/C. Forcados):
Effector T cells equipped with engineered antigen receptors specific for cancer targets have proven to be very efficient. Two methods have emerged: the Chimeric Antigen Receptors (CARs) and T-cell Receptor (TCR) redirection. Although very potent, CAR recognition is limited to membrane antigens which represent around 1% of the total proteins expressed, whereas TCRs have the advantage of targeting any peptide resulting from cellular protein degradation. However, TCRs depend on heavy signalling machinery only present in T cells which restricts the type of eligible therapeutic cells. Hence, an introduced therapeutic TCR will compete with the endogenous TCR for the signalling proteins and carries the potential risk of mixed dimer formation giving rise to a new TCR with unpredictable specificity. We have fused a soluble TCR construct to a CAR-signalling tail and named the final product TCR-CAR. In this project we prove that, if expressed, the TCR-CAR conserved the specificity and the functionality of the original TCR. In addition, we demonstrate that TCR-CAR redirection was not restricted to T cells. Indeed, after transduction, the NK cell line NK-92 became TCR positive and reacted against pMHC target. This opens therapeutic avenues combing the killing efficiency of NK cells with the diversified target recognition of TCRs.
You can learn more here and here.
CD37 CAR
CD37 CAR (S. Wälchli/B. Caulier):
We design novel CARs that target B-cell malignancies. CD37 is a tetraspanin that is widely expressed on the surface of mature B cells. Due to its high level expression across all subtypes of B-cell non-Hodgkin lymphoma (B-cell NHL), CD37 is one of many potential antibody targets for B-cell malignancies. Since adoptive immunotherapy using CAR gene-modified T-cells has generated impressive clinical responses in B-cell malignancies, we designed a novel second-generation CAR that redirects T-cell specificity towards CD37. Initial testing showed that CD37-directed human peripheral blood CAR T cells potently killed CD37+ B-cell NHL cell lines such as SU-DHL-4, BL-41, Mino and U-2932. We compared the anti-lymphoma activity of CD37-specific CAR T cells with that of CD19-specific CAR T cells and found no difference except for U-2932 cells which were more susceptible to CD37-specific CAR T cells, in concordance with high CD37 expression and low CD19 expression in these cells. The cell line U-2932 was originally derived from a patient with ABC-type of diffuse large B-cell lymphoma (DLBCL), who suffered from many relapses after multiple chemo- and radiotherapy regimens. Assessment of expression of CD19 and CD37 as well as other B-cell antigens such as CD20, CD22 and CD23 demonstrated a dramatic variation in their expression across the B-cell NHL cell lines. Genetic heterogeneity is common in B-cell NHLs and loss of or reduced expression of CD19 has already been reported in some B-cell NHLs. Moreover, extensive chemo- and radiotherapy regimens could potentially contribute to the loss of or reduced CD19 expression. Therefore, we are now in the process of screening B-cell NHL patient tumors for loss of or reduced expression of particular B-cell antigens, including CD19, CD20 and CD37. This type of screening can help us to identify the patient groups that are likely to benefit from CD19-, CD20- or CD37-targeted therapies. In summary, our findings suggest that CD37-directed CAR T cells can be used as an alternative to CD19-targeted CAR T cells, especially when CD19 expression is lost or reduced in patients’ tumor cells.
If you want to learn more about our CAR CD37 and its potential commercial applications, make sure to visit our Technical Transfer Office INVEN2 as well as this short presentation.
CLINICAL TRIALS
Cancer is a common disease causing significant impact in every nation via mortality and morbidity. Worldwide, more than 14 million new cases of cancer are diagnosed every year and over 8 million people die from cancer yearly. The most common types of cancer are lung cancer, female breast cancer, bowel and prostate cancers. The lifetime risk of developing cancer is ~42% for males and ~38% for females.
A great proportion of these cancers are ill- or not treatable by standard treatment options. Adoptive cell transfer (ACT), is often referred to as the next generation cancer therapy and its success in various trials has been impressive. Recently, on 30.08.2017, Kymriah (tisagenlecleucel) was approved by the FDA for certain groups of patients with a form of acute lymphoblastic leukemia (ALL). This is the first in history approval of a CAR-T cell therapy. Subsequently, on 18.10.2017, Yescarta (axicabtagene ciloleucel), also a CAR-T cell therapy, was FDA approved for patients with certain types of large B-cell lymphoma. To date most successful clinical trials have targeted hematological malignancies and treatment of solid tumors by this approach has been challenging for many reasons.
Our goal is to use our bench experience to define the major criteria that must be met to create a reliable, safe, and effective platform for adoptive T cell therapy. We want to utilize this platform to create therapy that can be deployed against a broad range of tumors, solid as well as hematological. The major goals of any immunotherapy is to combine a specific therapy with minimal toxic side effects in addition to the establishment of a durable clinical response and an immune memory in advanced cancers. We study not only CAR-T cells, but also TCR based adoptive therapy and dendritic cell vaccine therapies. Many projects are still at early stages, but some are entering clinical trials.
Our current clinical focus is on two tumor-targeting TCRs that were isolated from long-term survivors after cancer vaccination (Adoptive T-Cell therapy). The longevity of the TCRs in these patients without side effects makes them especially suitable for clinical trials. The two tumor antigens we are targeting are the universal antigen telomerase and the public neoantigen derived from TGFβRII frameshift mutant. Telomerase is very important in oncogenesis and 80-90% of all cancer cells have reactivation or up-regulation of telomerase. TGFβRII mutations are common in colorectal, gastric, pancreatic, biliary tract, lung and brain cancers. Clinical trials for both targets are underway. We have other potential candidate targets in the pipeline and we will be mentioning those here later as our work progresses.
Translational Research Unit
The Research lab (Group leaders: E.M. Inderberg and S. Wälchli) relies on the clinical trials patient biobank established by G. Gaudernack (Prof. emeritus, mentor). This represents an inestimable source of information about the patients’ response to immunological treatments over the years. Furthermore, the patient material can be re-analyzed and therapeutic molecules isolated. Indeed, selected material from “good responders” (cured patients) is characterized by molecular biology techniques and studied to reach the pre-clinical stage (in vivo assays). In addition, the scientific team is also developing novel tools to improve immunotherapy. The research lab can undertake basic studies independently. It possesses a fully functional molecular biology lab, cell culture hoods including a retroviral one, different high tech analysis instruments (flow cytometer, fluorescent microscope) and the access to the core facilities of the ICR including the animal facility.
The research lab has the capacity to undertake complete pre-clinical studies for immunotherapeutic molecules such as vaccines and adoptive T cell transfer (Universal Killer). It possesses the competence to deliver molecular biology products isolated from patient material (Adoptive T-cell Therapy), to perform cellular assays and in vivo animal studies. Finally, innovative tools are also developed in collaboration with academy (CAR) and private companies.
We are located at the OCCI
ADOPTIVE T-CELL THERAPY (UNIVERSAL KILLERS)
Ideally, efficient tumour-specific effector and memory T cells can be induced by therapeutic vaccination. Nevertheless, in certain cases, active immunization is difficult due to the lack of an effective endogenous T-cell repertoire against the tumour antigens targeted.
T cells can recognize peptides derived from all parts of the cell, including nuclear proteins, which greatly expands the number of potential targets in the tumour cells and offers a large number of new therapeutic opportunities.
Adoptive cell therapy (ACT) involves the administration of large number of highly selected cells with high avidity for tumour antigens.
T cells can be programmed and activated ex vivo to exhibit anti-tumour functions. These cells occur naturally in cancer patients, but are inhibited by numerous immunosuppressive mechanisms in vivo. Adoptive transfer of tumour-specific T cells from tumour infiltrating lymphocytes (TIL) expanded in vitro has already been shown to induce objective cancer regression in patients with metastatic melanoma. A limitation of this approach is the requirement for pre-existing tumour-reactive cells that can be expanded ex vivo and TILs can only be reliably grown from patients with a very limited number of cancer types and mainly melanoma.
In the absence of an adequate level of endogenous tumour-reactive T-cell response, recent clinical studies have shown that it is feasible to compensate for this by engineering a tumour-specific T-cell repertoire by the transfer of genes encoding TCRs or chimeric antigen receptors (CARs). Both of these methods are developed in-house (see below).
ADOPTIVE T-CELL THERAPY (CAR)
Ideally, efficient tumour-specific effector and memory T cells can be induced by therapeutic vaccination. Nevertheless, in certain cases, active immunization is difficult due to the lack of an effective endogenous T-cell repertoire against the tumour antigens targeted.
T cells can recognize peptides derived from all parts of the cell, including nuclear proteins, which greatly expands the number of potential targets in the tumour cells and offers a large number of new therapeutic opportunities.
Adoptive cell therapy (ACT) involves the administration of large number of highly selected cells with high avidity for tumour antigens.
T cells can be programmed and activated ex vivo to exhibit anti-tumour functions. These cells occur naturally in cancer patients, but are inhibited by numerous immunosuppressive mechanisms in vivo. Adoptive transfer of tumour-specific T cells from tumour infiltrating lymphocytes (TIL) expanded in vitro has already been shown to induce objective cancer regression in patients with metastatic melanoma. A limitation of this approach is the requirement for pre-existing tumour-reactive cells that can be expanded ex vivo and TILs can only be reliably grown from patients with a very limited number of cancer types and mainly melanoma.
In the absence of an adequate level of endogenous tumour-reactive T-cell response, recent clinical studies have shown that it is feasible to compensate for this by engineering a tumour-specific T-cell repertoire by the transfer of genes encoding TCRs or chimeric antigen receptors (CARs). Both of these methods are developed in-house (see below).
ADOPTIVE T-CELL THERAPY (TCR)
Ideally, efficient tumour-specific effector and memory T cells can be induced by therapeutic vaccination. Nevertheless, in certain cases, active immunization is difficult due to the lack of an effective endogenous T-cell repertoire against the tumour antigens targeted.
T cells can recognize peptides derived from all parts of the cell, including nuclear proteins, which greatly expands the number of potential targets in the tumour cells and offers a large number of new therapeutic opportunities.
Adoptive cell therapy (ACT) involves the administration of large number of highly selected cells with high avidity for tumour antigens.
T cells can be programmed and activated ex vivo to exhibit anti-tumour functions. These cells occur naturally in cancer patients, but are inhibited by numerous immunosuppressive mechanisms in vivo. Adoptive transfer of tumour-specific T cells from tumour infiltrating lymphocytes (TIL) expanded in vitro has already been shown to induce objective cancer regression in patients with metastatic melanoma. A limitation of this approach is the requirement for pre-existing tumour-reactive cells that can be expanded ex vivo and TILs can only be reliably grown from patients with a very limited number of cancer types and mainly melanoma.
In the absence of an adequate level of endogenous tumour-reactive T-cell response, recent clinical studies have shown that it is feasible to compensate for this by engineering a tumour-specific T-cell repertoire by the transfer of genes encoding TCRs or chimeric antigen receptors (CARs). Both of these methods are developed in-house (see below).
