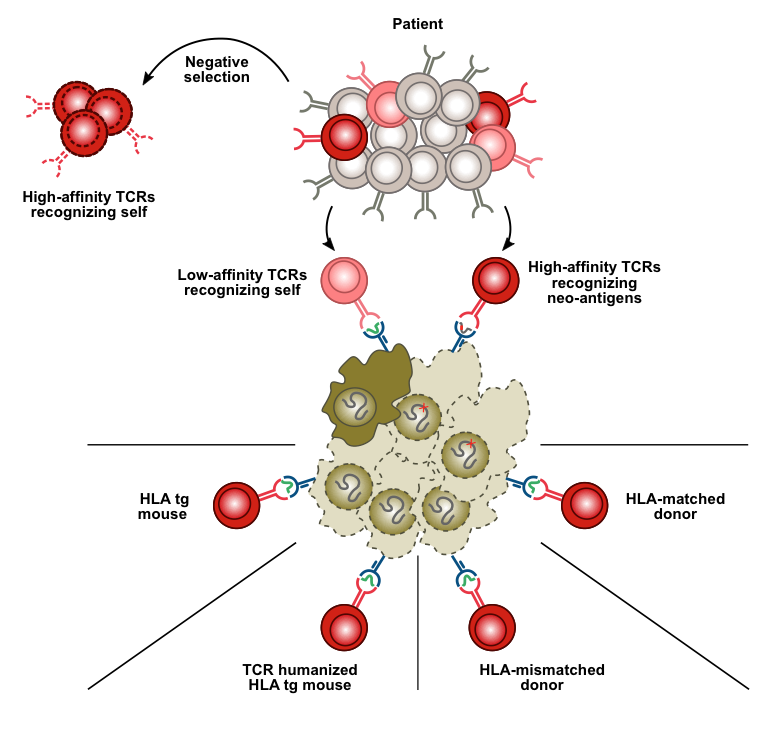
Research area 2: Targeting of self-antigens
Therapeutic antibodies recognize "normal" cell surface proteins with cell type-restricted expression. A good example is antibodies to the B-cell antigen CD20, which are used for treatment of leukemia and lymphoma. These antibodies kill normal and malignant B cells indiscriminately. Yet, the ability to limit cytotoxicity to a single cell type represents an important step forward compared to chemotherapy and radiation therapy. The major advantage of self-antigens as therapeutic targets (relative to cancer mutations) is that the same target can be used in all patients with a particular cancer type. Similarly, CARs (chimeric antigen receptors) target normal cell surface proteins like CD19 to which the antibody portion of the receptor binds. Adoptive cell therapy using T-cells genetically equipped with CARs has been a tremendous success in the treatment of B-lymphoid leukemia and lymphoma. In spite of worldwide efforts it has, however, proven difficult to find similarly good targets in other leukemias, and in other cancer types. The number of candidate self-antigens that could be targeted in cancer can be increased dramatically with the use of T-cell receptors as alternatives to CARs in adoptive cell therapy. The reason is that T cells, in contrast to CARs/antibodies, have the ability to recognize peptides from intracellular proteins, and the large majority of cellular proteins are inside the cell. A major focus in our group is to direct T-cell receptors to peptides derived from self-antigens expressed on cancer cells by use of foreign (allogeneic) HLA for antigen presentation.
Project
Using T cell receptors from “non-tolerized” donors to target self-antigens
To overcome immunological self-tolerance for "normal" proteins, our laboratory has taken a new approach that involves peptide presentation on foreign HLA molecules. We have shown that it is feasible to obtain T cells from one individual that selectively kill defined cell types from another individual with a different tissue type. The recognition elements are peptides from "normal" proteins with cell type-restricted expression, bound to a tissue type molecule of the target individual. A multitude of novel targets and their cognate T-cell receptors have been isolated from healthy donors using our patent-pending technology. T cells engineered to express receptors recognizing these targets are expected to be useful to treat subtypes of leukemia or lymphoma in all patients who share the same tissue type molecule (HLA-A2; shared by 50% of Caucasians).
Selected references
T cells targeted to TdT kill leukemic lymphoblasts while sparing normal lymphocytes
Nat Biotechnol
DOI 10.1038/s41587-021-01089-x, PubMed 34873326
T cells raised against allogeneic HLA-A2/CD20 kill primary follicular lymphoma and acute lymphoblastic leukemia cells
Int J Cancer, 130 (8), 1821-32
PubMed 21630262
Targeting B cell leukemia with highly specific allogeneic T cells with a public recognition motif
Leukemia, 24 (11), 1901-9
PubMed 20844564
Dendritic cells engineered to express defined allo-HLA peptide complexes induce antigen-specific cytotoxic T cells efficiently killing tumour cells
Scand J Immunol, 69 (4), 319-28
PubMed 19284496
Targeting B-cell neoplasia with T-cell receptors recognizing a CD20-derived peptide on patient-specific HLA
Oncoimmunology, 5 (5), e1138199
PubMed 27467957
