Hormone treatment resistance in ER+ breast cancer
ER+ breast tumours undergo genome-wide epigenetic alterations, and we have previously shown that enhancer methylation results in oncogenic estrogen signalling (published in Fleischer et al., Genome Biol 2014, Fleischer & Tekpli et al., Nat Commun 2017, and Ankill et al., NAR Cancer 2022).
Altered DNA methylation and chromatin interactions are crucial in the development of resistance to hormone therapy; a key treatment plan for post-menopausal ER+ breast cancer patients. We aim to identify and characterize treatment resistance-driving epigenetic alterations found in human breast tumour samples using single-cell ATAC-seq. Single-cell ATAC-seq explores the evolving epigenetic landscapes of both tumour and non-tumour cell populations of a patient biopsy (Figure 1), offering novel insight into cell biology, disease etiology and treatment response.
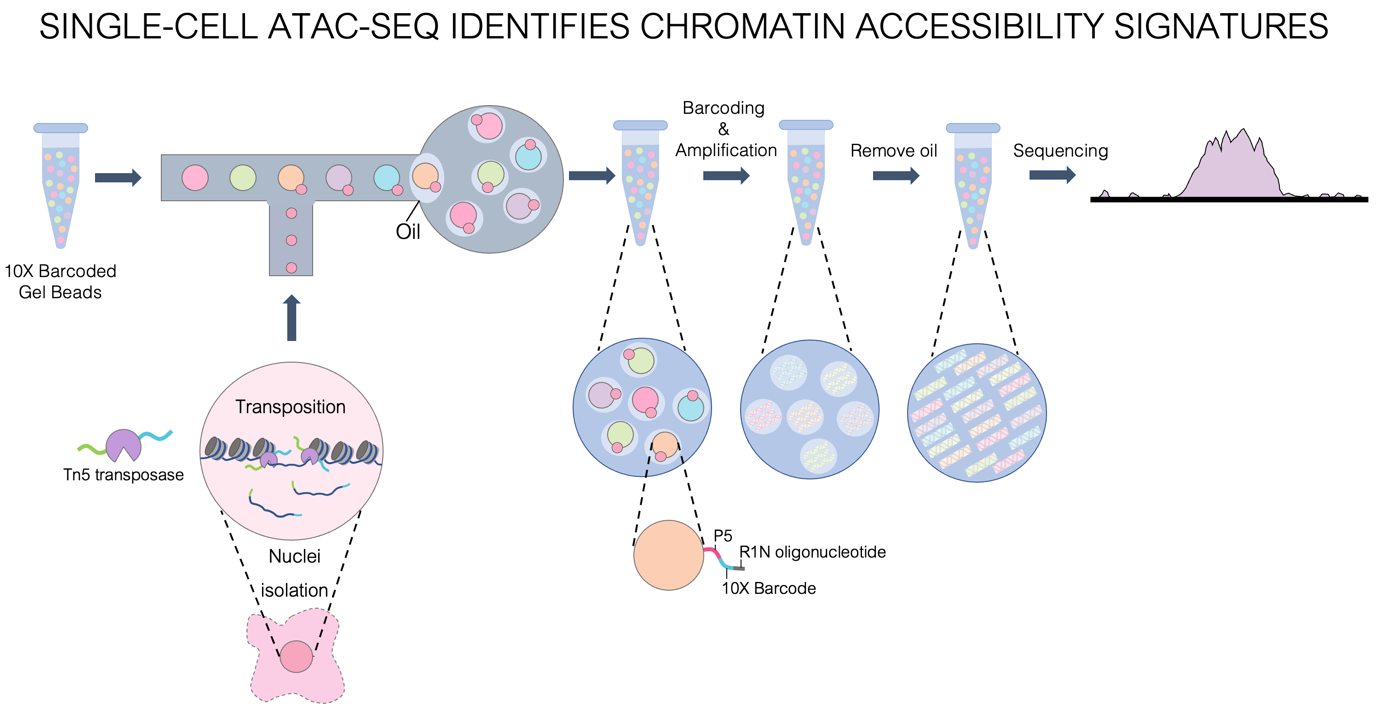
Figure 1. Single-cell ATAC-seq identifies open chromatin regions of the genome. First, nuclei are extracted from cells or tissue samples. Then, during the tagmentation step, a hyperactive Tn5 transposase enzyme will simultaneously cleave and insert adapters into open chromatin DNA regions of the genome. The nuclei are separated and mixed with unique 10X barcoded gel beads in a microfluidics chamber – giving each cell its unique barcode ID. Following amplification and purification, the barcoded DNA fragments are sequenced, resulting in a genome-wide map of open chromatin areas in the cell. Figure adapted from 10X Genomics (www.10xgenomics.com).
We are studying the epigenome of patient biopsies obtained from the NeoLetExe clinical trial; a cross-over trial assessing the effect of aromatase inhibitors letrozole (Let) and exemestane (Exe) in post-menopausal locally advanced ER+ patients (Figure 2). Patients are treated with either Let-Exe or Exe-Let for two months each and biopsies are taken at three timepoints; (1) at the start of the trial, (2) at the drug crossover point, and (3) after completing the hormone treatment at the point of surgery. We will characterise the intrinsic and acquired epigenetic properties of responding and non-responding tumours from NeoLetExe biopsies as participants undergo hormonal treatment.
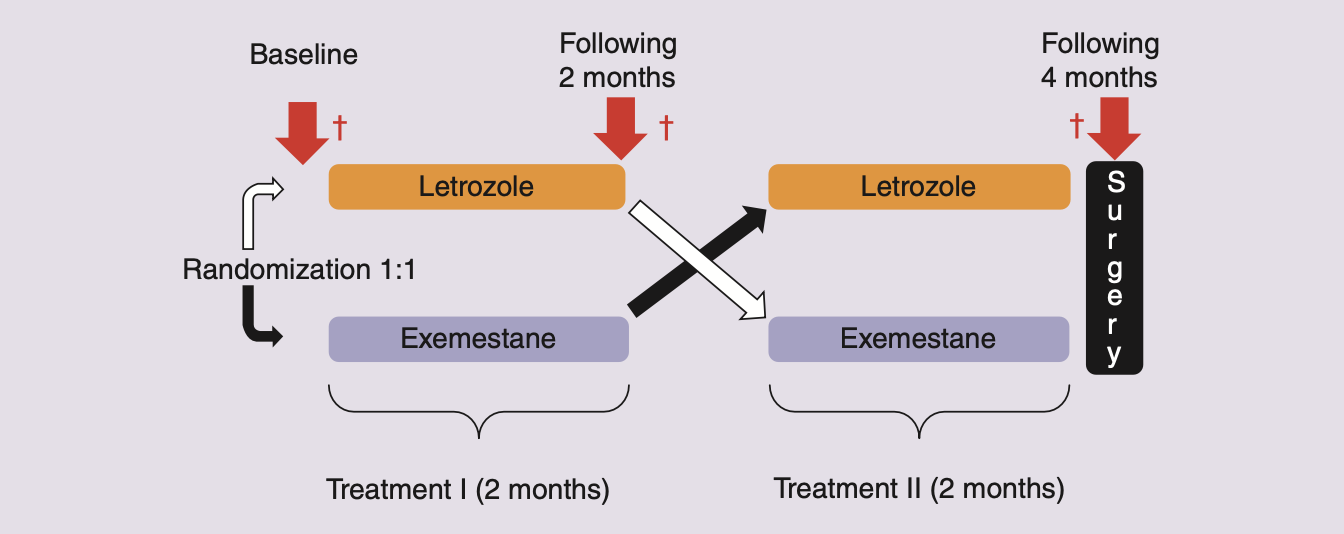
Figure 2. Overview of the NeoLetExe clinical trial. The NeoLetExe clinical trial is a cross-over trial assessing the effect of aromatase inhibitors letrozole (Let) and exemestane (Exe) in post-menopausal locally advanced ER+ patients. Patients are treated with either Let-Exe or Exe-Let for 2 months each and biopsies are taken at these 3 timepoints. Figure is taken from Bahrami et al., Future Oncol 2019.
People involved: Margrete Langmyhr, Xavier Tekpli, Vessela N. Kristensen, Anthony Mathelier, Thomas Fleischer
