MATRIX-RARE Study design

MATRIX-RARE is a prospective, non-randomized, multi-institutional clinical trial with a combined umbrella and basket design. It consists of a molecular screening phase and a treatment phase. Patients will be enrolled in treatment cohorts based on tumour type and the tumour’s molecular profile.
Biological material including tumour tissue and blood samples will be collected prior to treatment start, during treatment and upon progression. Molecular profiling is performed using TSO500 (Illumina), a next-generation sequencing assay that enables genomic profiling of the most important DNA and RNA variants implicated in various tumour types, in addition to key current and emerging immunotherapy biomarkers like microsatellite instability and tumour mutational burden.
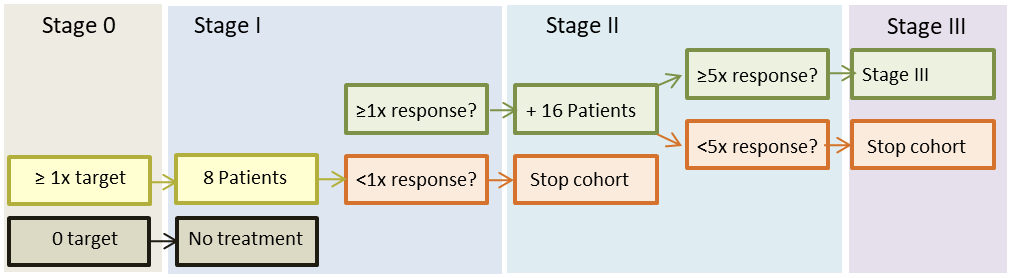
Stage I cohorts will be considered positive if ≥1 patient show objective response or stable disease at 16 weeks. Sixteen more patients will then be included. If ≥5 patients in stage II cohorts show objective response or stable disease after 16 weeks, the cohort may proceed to stage III, after consultation with the manufacturer and the health authorities. The aim is then to confirm the response rate (CR/PR/SD) that was observed in stage II, with a statistical power of 80-95% (depending on the clinical benefit rate observed in stage II).
