Prenatal Opioid Exposure
Over the past two decades, there has been a significant increase in heroin usage. Opioid maintenance treatment with methadone or buprenorphine is the recommended approach for treating heroin addiction, even during pregnancy. However, both clinical and preclinical studies have highlighted potential adverse short- and long-term consequences for children exposed to this treatment during fetal life.
The main aim of this project is to investigate whether there is a causal relationship between the use of methadone or buprenorphine during pregnancy and the negative effects reported in children.
To study this, we have established a rat model that includes prenatal opioid exposure and reduced maternal care in the first period after birth, aiming to simulate the human situation of opioid-dependent mothers and their children. We examine two generations of offspring at different developmental stages, from newborn to adulthood, using behavioral tests to obtain information about cognitive, emotional and social functioning. We also study the pharmacokinetics of the administered drugs, neonatal birth outcomes, and drug-induced cellular and genetic changes.
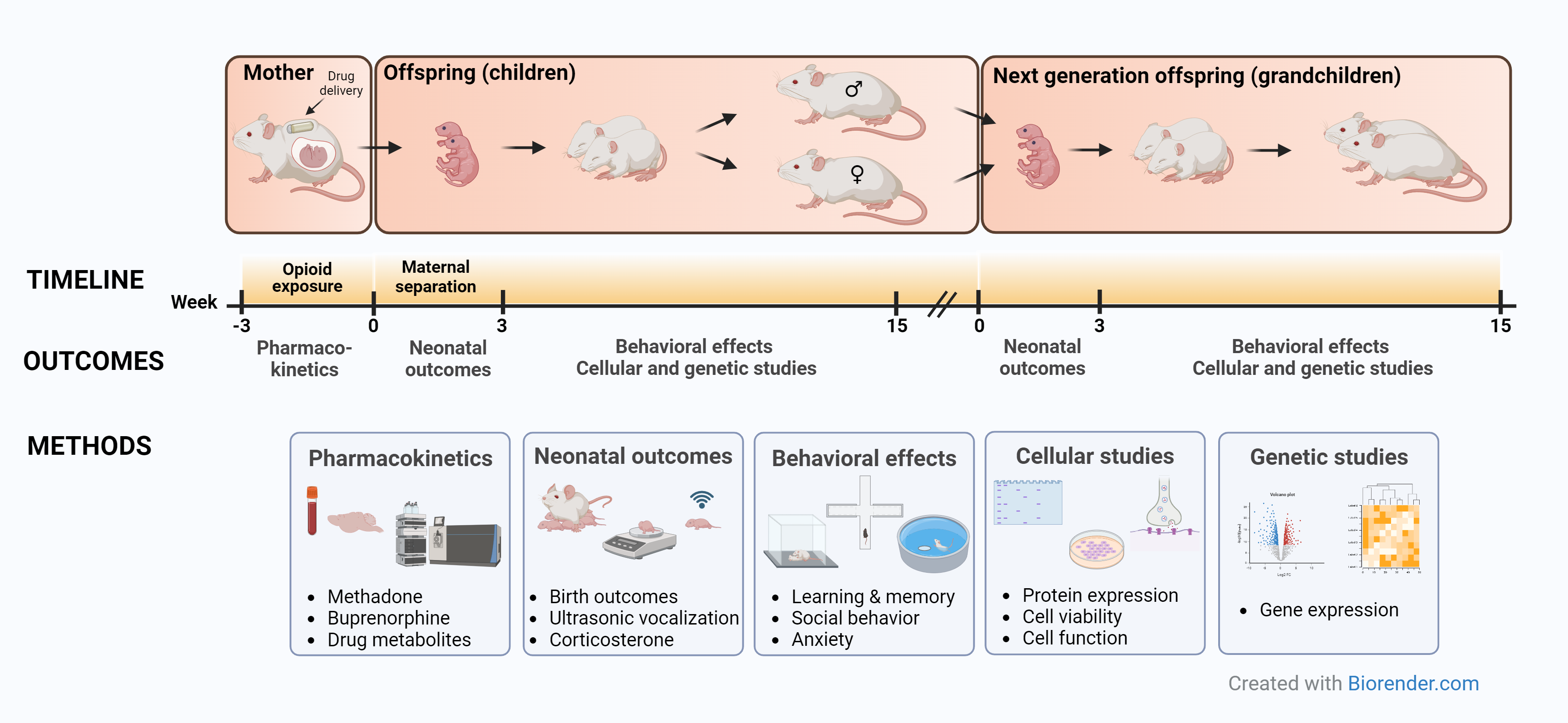
Research Questions
- Does prenatal exposure to methadone or buprenorphine impair learning and memory?
- How does prenatal exposure to methadone or buprenorphine influence social behavior and communication in young and adult offspring?
- Does prenatal exposure to methadone or buprenorphine lead to stress or anxiety?
- How does prenatal exposure to methadone or buprenorphine alter the expression of genes involved in brain development and function?
- Does prenatal exposure to methadone or buprenorphine affect males and females differently?
- Are the methadone- or buprenorphine-induced changes in the offspring inherited by subsequent generations?
If you have questions about the project or suggestions for research collaboration, please contact Jannike Mørch Andersen (jannike.morch.andersen@ous-hf.no) at the Section for Drug Abuse Research, Department of Forensic Sciences, Oslo University Hospital, Norway.
Publications
Nygaard E, Andersen JM (2023)
Prenatal exposure to opioids
In Brown GG, King TZ, Haaland KY, Crosson B (Eds.) APA handbook of neuropsychology, Neurobehavioral disorders and conditions: Accepted science and open questions, vol. 1, 649-667
https://doi.org/10.1037/0000307-030
Andersen JM, Høiseth G, Nygaard E (2020)
Prenatal exposure to methadone or buprenorphine and long-term outcomes: A meta-analysis
Early Hum Dev, 143, 104997
DOI 10.1016/j.earlhumdev.2020.104997, PubMed 32146140
Kongstorp M, Bogen IL, Steinsland S, Nerem E, Salih TW, Stiris T, Andersen JM (2020)
Prenatal exposure to methadone or buprenorphine alters µ-opioid receptor binding and downstream signaling in the rat brain
Int J Dev Neurosci, 80 (5), 443-453
DOI 10.1002/jdn.10043, PubMed 32484968
Kongstorp M, Bogen IL, Stiris T, Andersen JM (2020)
Prenatal exposure to methadone or buprenorphine impairs cognitive performance in young adult rats
Drug Alcohol Depend, 212, 108008
DOI 10.1016/j.drugalcdep.2020.108008, PubMed 32402939
Fjelldal MF, Hadera MG, Kongstorp M, Austdal LPE, Šulović A, Andersen JM, Paulsen RE (2019)
Opioid receptor-mediated changes in the NMDA receptor in developing rat and chicken
Int J Dev Neurosci, 78, 19-27
DOI 10.1016/j.ijdevneu.2019.07.009, PubMed 31351113
Kongstorp M, Bogen IL, Stiris T, Andersen JM (2019)
High Accumulation of Methadone Compared with Buprenorphine in Fetal Rat Brain after Maternal Exposure
J Pharmacol Exp Ther, 371 (1), 130-137
DOI 10.1124/jpet.119.259531, PubMed 31358559
Andersen JM (2017)
Eksperimentelle studier i forsøksdyr. Hva vet vi om langvarige effekter i avkom eksponert for metadon eller buprenorfin i fosterlivet? Finnes det relevante epigenetiske studier? Kunnskapsoppsummering på oppdrag fra Helsedirektoratet.
Mørch-Jonsen GH, Andersen JM, Høiseth G (2015)
Rapport om konsekvenser for fosteret/barnet ved bruk av rusmidler/avhengighetsskapende legemidler i svangerskapet
Utarbeidet av Nasjonalt folkehelseinstitutt på oppdrag fra Helsedirektoratet, IS-2438
Nasjonale faglige retningslinjer - rapportmal (helsedirektoratet.no)
