Background Information
Intracellular Signalling
Intracellular signalling is characterised by spatial and temporal integration of multiple signalling cascades into signalling networks. Our group especially studying the T cell receptor (TCR) signalling in cancer and infections. The proximal TCR signalling is complex, multi-protein and well-orchestrated event (figure). Engagement of CD3 along with CD2 and/or CD2 results in differential signalling patterns. There are several proximal signalling events critical for successful TCR signalling.
One of our projects is aimed at studing the role of common second messenger cyclic adenosine monophosphate (cAMP) and scaffolding protein A-kinase anchoring proteins (AKAPs) in numerous cellular processes.
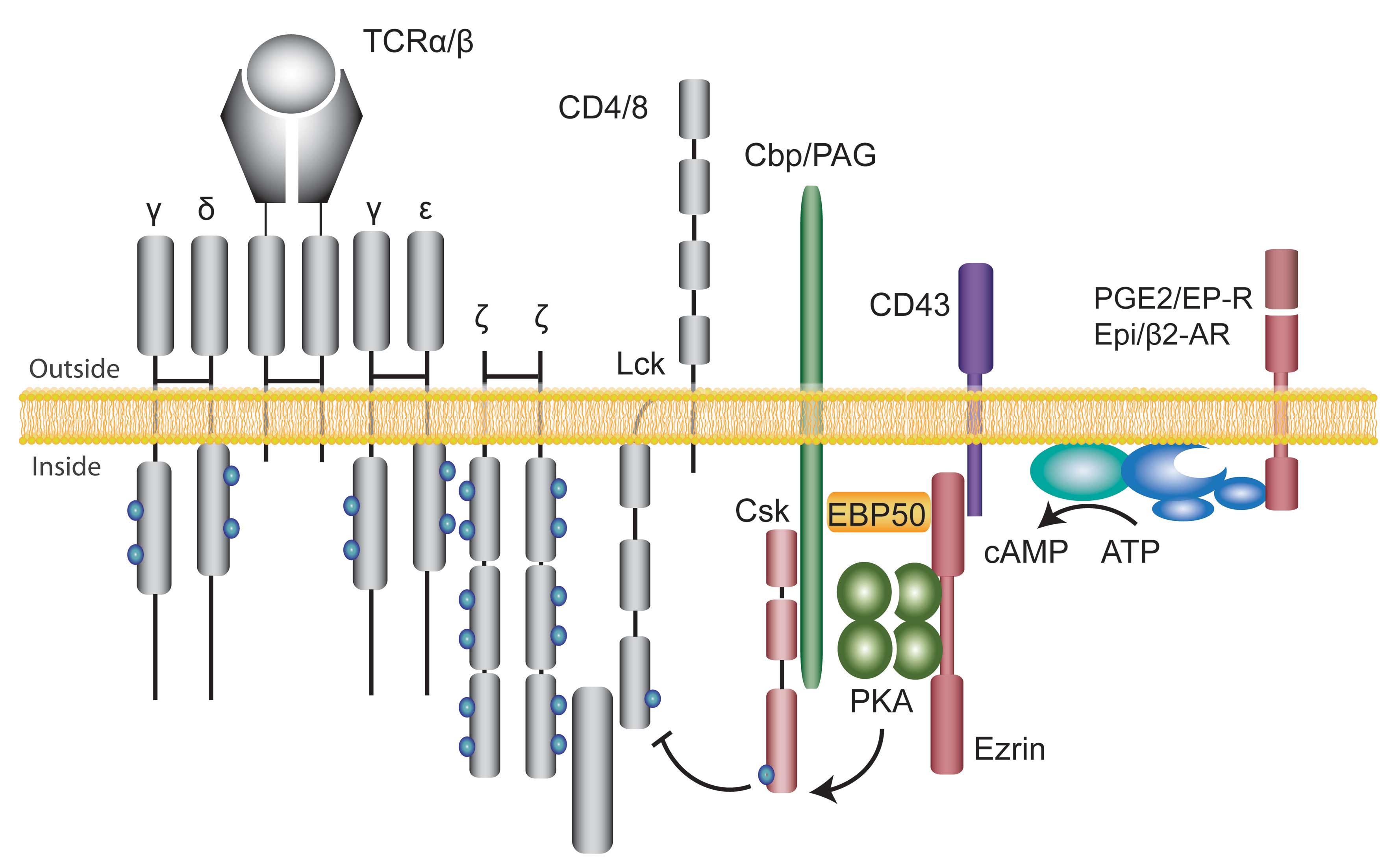
Cyclic AMP has been implicated in signalling downstream of a large number of hormones, neurotransmitters, prostaglandins and chemokines and is known to be a potent inhibitor of T cell proliferation and cytokine production. The biological effects of cAMP are mainly mediated by the activation of cAMP-dependent protein kinase A (PKA). AKAPs both facilitate and localise signalling within the cell by assembling the signalling elements in close proximity to each other. It is estimated that there are more than 75 AKAP proteins, 50 of which have thus far been characterised. Although multiple receptors and targets use cAMP as a second messenger, signalling specificity is obtained due to targeting of PKA to sub-cellular compartments through interaction with AKAPs.
Regulatory T cells
Human regulatory T cells (Tregs) comprise 5-10% of the peripheral CD4+ T cell pool and are recognised by constitutive expression of CD25 (IL-2Rα) and the transcription factor FOXP3. Tregs are functionally characterised by their ability to suppress effector T cells and they play an essential role in maintaining peripheral immunological tolerance.
Tregs can be naturally occurring derived from the thymus (nTregs) and adaptive or peripherally induced Tregs (iTregs) originate from naïve T cells or effector T cells that undergo a phenotypic switch during T cell activation and differentiation. Both nTregs and iTregs are involved in suppression of effector T cells directly or indirectly (see figure).
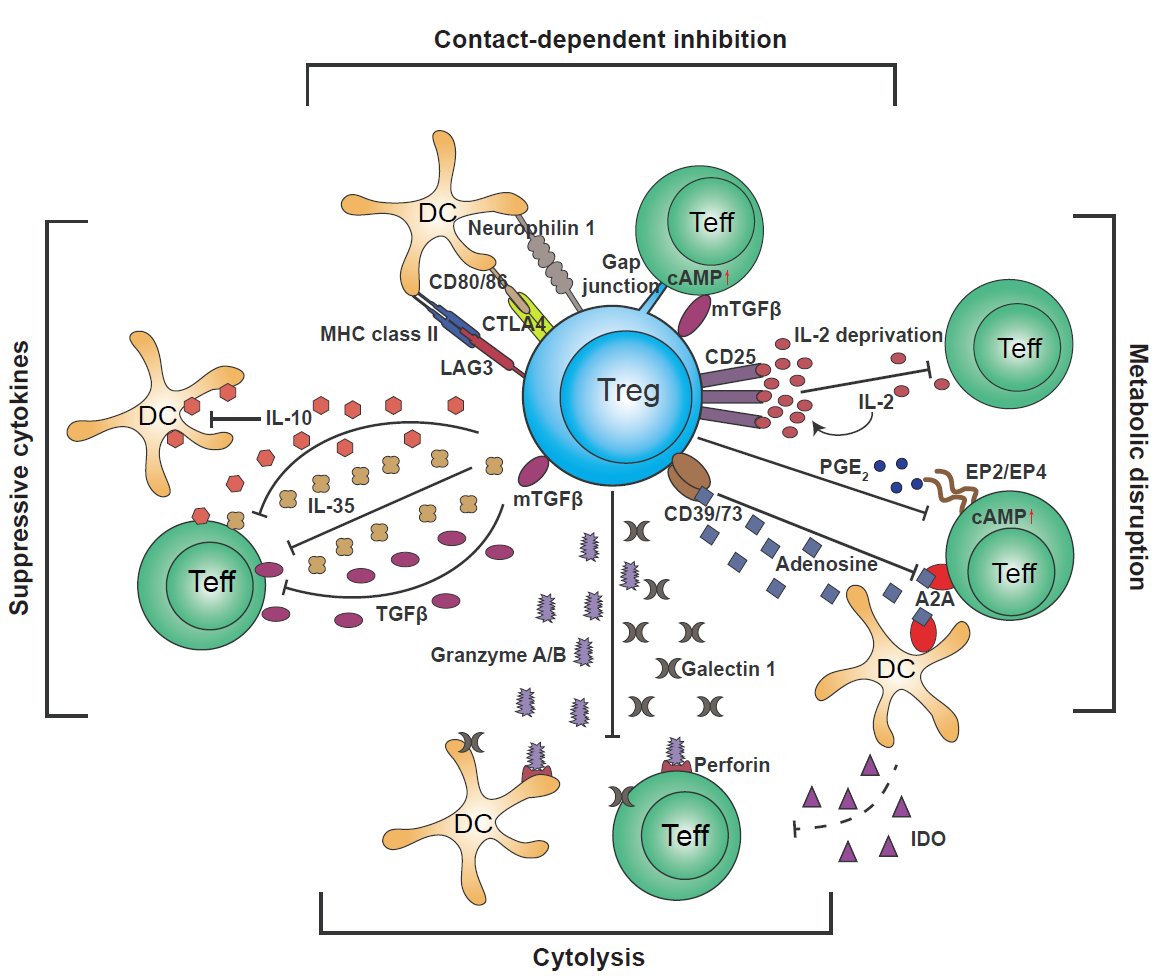
Putative mechanisms used by regulatory T cells: Targeting DCs – modulation of antigen-presenting cell activity through Treg engagement of co-stimulatory receptors on the DC surface, leading to weak or abrogated signals to naïve/effector T cells; Metabolic disruption – includes cytokine deprivation, cyclic AMP-mediated inhibition, and adenosine receptor (A2A)-mediated immunosuppression; Competition – for critical cytokines, such as IL-2, or direct disruption of effector cell engagement with APCs; Cytolysis – direct cytotoxic effect through the production of Granzyme B and Perforin and consequent apoptosis of effector T cells or APCs; Production of inhibitory cytokines – including IL-10, IL-35, and TGF-β.
iTregs acquire immunosuppressive properties at the site of antigen exposure, are short-lived and considered to represent an important mechanism that turns off the immune response after an acute infection in addition to limit the immune reactivity during chronic immune responses to prevent immune-related tissue destruction. However, in cancer the induction of iTregs that inhibit anti-tumor immunity is a dysfunctional response. nTregs, on the other hand, represent a stable T cell phenotype and are considered to be a key for maintaining peripheral immune self-tolerance.
