Kay Oliver Schink identifies a new regulator of macropinocytosis, an important mechanism for nutrient acquisition by cancer cells

In a recent article in Nature Communications, published online on the 12 of November, 2021, project group leader Kay Oliver Schink and his coworkers in Harald Stenmark’s group at the Institute for Cancer Research and the Centre for Cancer Cell Reprogramming (CanCell) identify a new mechanism how the protein Phafin2 can regulate a process called “macropinocytosis”.
Macropinocytosis – also called “cellular drinking” - is a cellular uptake mechanism. Cells use their actin cytoskeleton to form large vesicles – macropinosomes – which allow cells to take up large amounts of extracellular fluid. This process is important for the immune system. Immune cells – especially dendritic cells, which are the “early warning system” of the body - use this mechanism to scan body fluids for foreign substances.
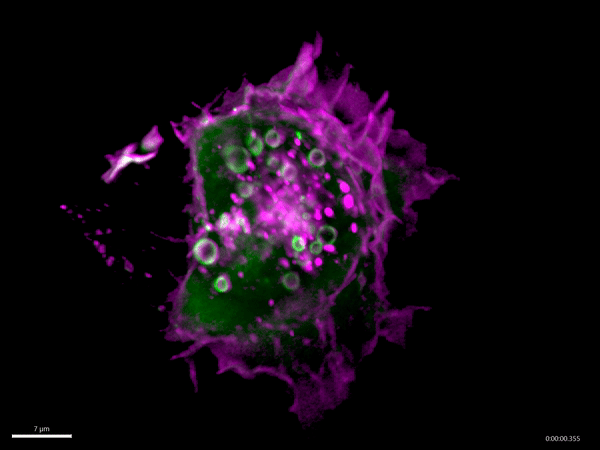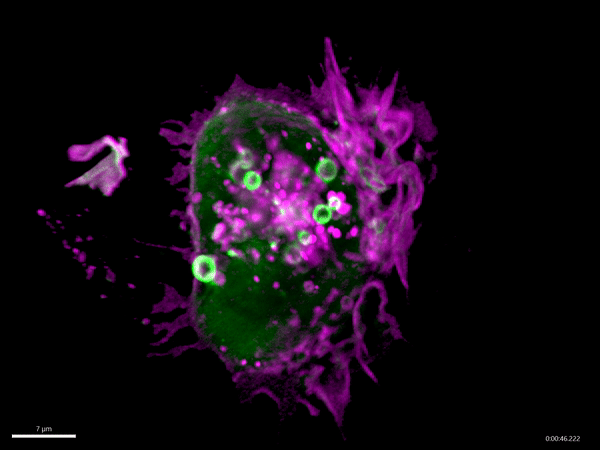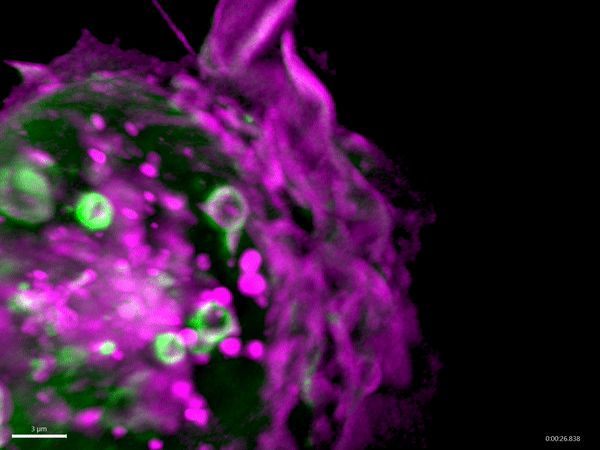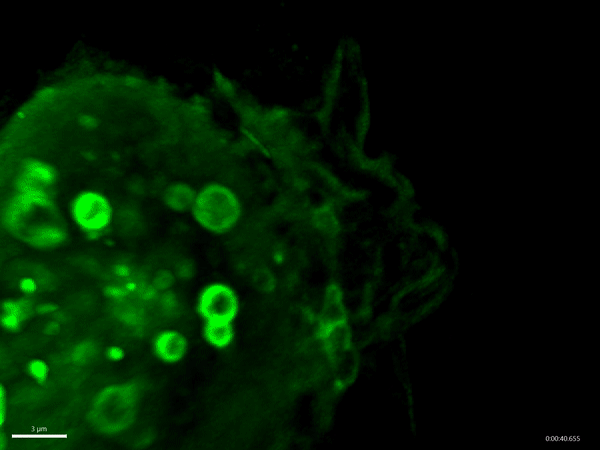
Macropinocytosis has recently gained much attention as a potential target for cancer treatment, as cancer cells use this process to gain additional nutrients to fuel their growth. Aggressive cancer types, such as pancreatic cancer, exploit macropinocytosis as a mechanism to take up additional nutrients. The fast growth of these cancers requires lots of energy and amino acids, which often become scarce within a tumor. To be able to grow quickly, these cells drink – by using macropinocytosis – the protein-rich extracellular fluids, which are then digested. The resulting amino acids are then used by the cancer cells to fuel their growth. Not only cancer cells exploit macropinocytosis. Also, many pathogens – for example Salmonella bacteria and viruses such as HIV and Ebola virus – exploit macropinocytosis to infect cells.
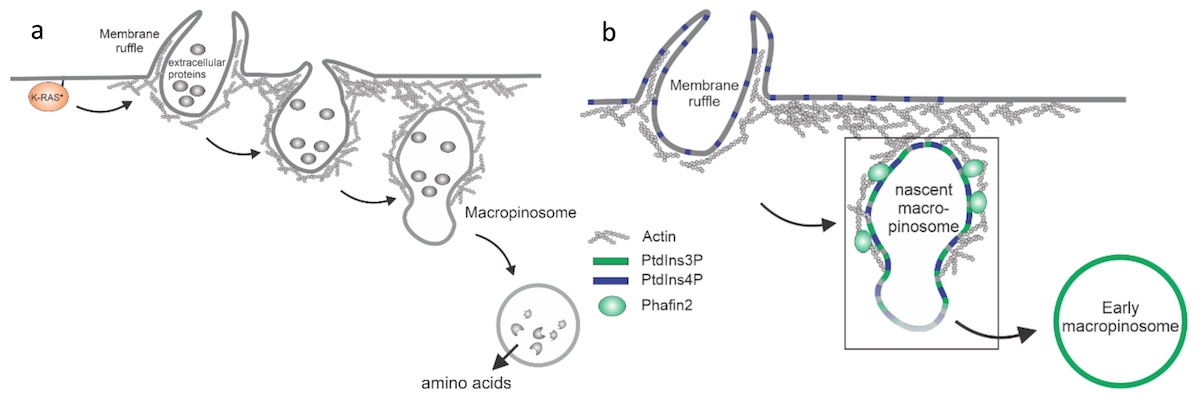
Schink and his co-workers have identified a new regulator of this process, the protein Phafin2. They find that Phafin2 is involved in very early events of macropinocytosis, directly after the macropinosome is formed at the surface of the cell. During this process, Phafin2 interacts with the actin cytoskeleton and allows the newly formed macropinosome to remove actin from its surface to fully enter the cell. Cells lacking Phafin2 show defects in macropinocytosis.
During their studies, the authors found that many cancer types show amplifications of Phafin2, which suggest that this gives cancer cells a growth advantage. To test this hypothesis, they used CRISPR/Cas9 to knock out Phafin2 in pancreatic cancer cells. The Phafin2 knockout cells are not able to use macropinocytosis and show impaired growth when nutrients are scarce.
As many of the highly aggressive RAS-transformed cancers – especially KRAS-transformed pancreatic cancers - show high levels of macropinocytosis, this process is an attractive target for cancer therapy, as disrupting the amino acid supply routes would slow cancer cell growth. Moreover, nanoparticles and other large drug conjugates are preferably taken up via macropinocytosis and identifying cancer cells with high or low levels of macropinocytosis could guide targeted therapy.
Schink’s project group is now searching for new regulators of macropinocytosis which can be potential drug targets to disrupt macropinocytosis. This could lead to new treatment approaches for cancer and for other diseases that exploit macropinocytosis, for example newly emerging viruses.
Links:
The Nature Communications article:
The phosphoinositide coincidence detector Phafin2 promotes macropinocytosis by coordinating actin organisation at forming macropinosomes
Kay Oliver Schink, Kia Wee Tan, Hélène Spangenberg, Domenica Martorana, Marte Sneeggen, Virginie Stévenin, Jost Enninga, Coen Campsteijn, Camilla Raiborg & Harald Stenmark
Nature Communications volume 12, Article number: 6577 (2021)
Home page of project group headed by Kay Oliver Schink:
Phosphoinositide control of early endocytic trafficking
Harald Stenmark's research group:
Cellular membrane dynamics
