Science Signalling paper from Taskén group: Systems analysis of prostaglandin E2 signaling networks through four GPCRs on T cells

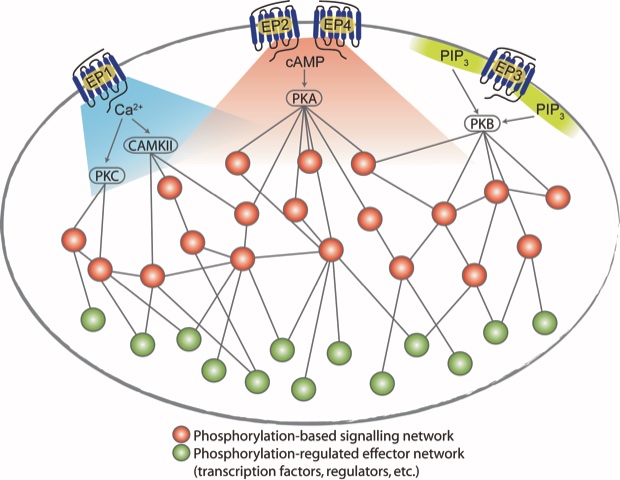
Immune suppressive mechanisms serve several important biological functions in the human body, which includes controlling autoimmunity and minimizing harmful side effects, inflammation and tissue damage in the course of healthy and successful immune responses to pathogens. These mechanisms, which prevent immunological overshoot, can be hijacked by growing tumor cells, resulting in tumor immune escape, which is a crucial process in tumorigenesis. The lipid mediator prostaglandin E2 suppresses antitumor immunity by activating its four related GPCRs (EP1 to EP4) on T cells.
While targeting immune escape mechanisms through immune checkpoint inhibitors has been very successful, they work only in immunologically hot tumors and only for a subgroup (40-50%) of patients. Thus, many of the other classes of tumor immune evasion mechanisms also appear to be acting in parallel and have clinical importance. Therapeutic strategies for blocking these mechanisms to rescue anti-tumor immunity (such as EP4 antagonists, now in clinical trials, see also below on ASAC trial at OUH) could add to the current repertoire of immunostimulating therapies in a precision immune oncology approach for patients not responding to immune checkpoint inhibitors.
In the present paper quantitative phosphoproteomics and phosphoflow cytometry was used to analyze downstream signaling elicited through the EP1, EP2, EP3 and EP4 GPCRs on T cells. By specific agonists and antagonists, the EP1-4 receptors were activated simultaneously or individually in different T cell subsets. The analysis and signal network modeling revealed more than 1,500 regulated phosphosites (of 5-8,000 in each cell type) and receptor specific and shared signaling pathways in all or only in individual subset of T cells. Mechanisms of cross-talk and signal integration were identified and the paper is classified as Research Resource for future explorations of functional consequences of PGE2 receptor–specific signaling. The work has been a great collaboration with the Albert Heck group at Utrecht Medical Centre (on phosphoproteomics) and the group of Julio Saez-Rodrigues at Heidelberg University on network modelling.
Link to paper:
Systems approach reveals distinct and shared signaling networks of the four PGE2 receptors in T cells
ANNA M. LONE, PIERO GIANSANTI, MARTHE JØNTVEDT JØRGENSEN, ENIO GJERGA, AURELIEN DUGOURD, ARJEN SCHOLTEN, JULIO SAEZ-RODRIGUEZ, ALBERT J. R. HECK AND KJETIL TASKÉN
SCIENCE SIGNALING - 5 Oct 2021 - Vol 14, Issue 703 - DOI: 10.1126/scisignal.abc8579
The ASAC trial (https://www.asac.no) is a placebo-controlled, randomized international multicenter trial examining the effect of treatment with acetylsalicylic acid (inhibiting prostaglandin production) in patients undergoing resection for liver metastasis from colorectal cancer, led from OUH.
See also paper in Trials on the ASAC protocol: https://doi.org/10.1186/s13063-021-05587-w
Other links:
