Bridging the gap to proteomics with new high-parameter instruments at the Flow Cytometry Core Facility
Highly multiplexed analysis of cell suspensions or tissue sections is now available at the Institute of Cancer Research. The Flow Cytometry Core facility has recently installed a 30-parameter fluorescence flow cytometer (FACSSymphony A5) and a 135-channel mass cytometer (Helios) with a state-of-the-art tissue imager (Hyperion). The possibility of interrogating more than 20 fluorescence-markers or more than 50 metal-markers simultaneously in millions of individual cells can unveil new cell types, functions and biomarkers for all research areas. The spatial context of tissue microenvironments across complex cellular phenotypes is empowered by the imaging capability of up to 37 protein, RNA and/or DNA markers simultaneously with 1 µm resolution. You can bring archival FFPE tissue and correlate the mass cytometry imaging with all the other data collected for those patients.
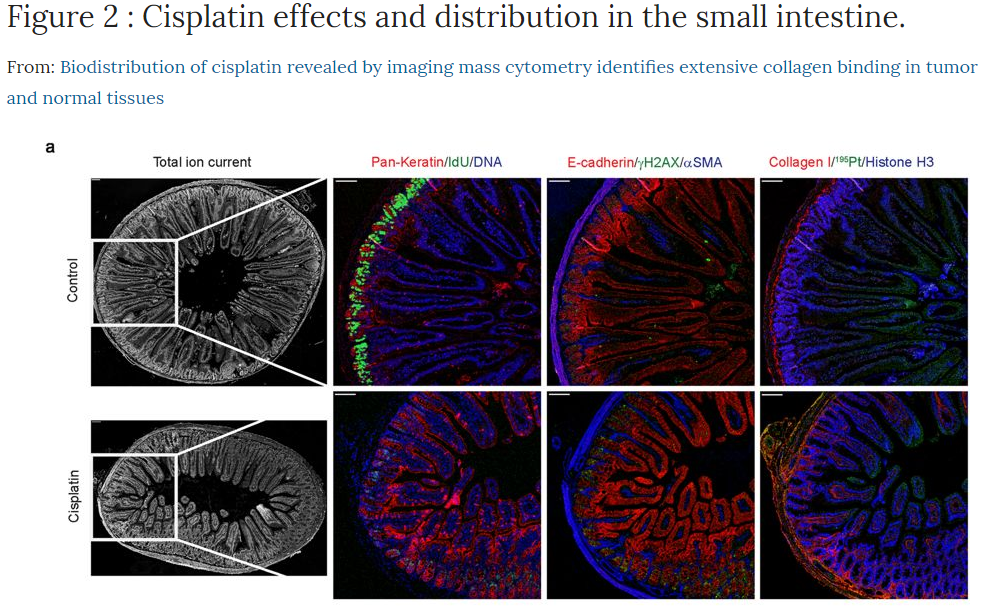
This summer the high-parameter capability at the Flow Cytometry Core Facility (FCCF) increased by the acquisition of the FACSymphony A5 (BD) and the Hyperion Imaging system (Fluidigm). The mass cytometer for cell suspension samples was upgraded from a CyTOF 2 to the 3rd generation instrument Helios, increasing the mass range to 75-209 Da. Our mass cytometer now offers 135 mass channels for analysis of metal-tagged antibodies or other probes. In addition, a revolution in histology is provided by the Hyperion Imaging system that connects to the Helios. The Hyperion uses a UV laser to ablate 1 µm2 pieces of tissue from slides, helium gas lifts those pieces into the mass cytometer for detection by time-of-flight mass spectrometry. Staining of formalin-fixed paraffin-embedded (FFPE) or frozen tissue sections simultaneously with up to 37 metal-tagged antibodies of high isotope purity enables deep interrogation of tissues and tumors at subcellular resolution. Computational analysis enables single cell data of your tissue for further downstream analysis. You can now interrogate such things as tumor heterogeneity, tumor microenvironment and/or immune infiltration with broad phenotyping of the activation states, in your archival FFPE material comparing it to the metadata and/or genomic information from the same patients/animal models. Tissue micro arrays with 37 markers can shed light on differential treatment response, outcome etc. Combinations of mass/flow cytometry on cell suspensions to discover rare populations with 30-50 markers and spatial localization with imaging of the corresponding tissue will provide the research environment at OUS with a powerful toolbox for new discoveries.
Providing the possibility of 28 fluorescence parameters from 5 high powered lasers, the FACSymphony at the Institute for Cancer Research is currently the most advanced analytical flow cytometer in Norway. The system has new electronics with minimal electronic noise. Lower noise and higher laser power enables better separated cell populations. In addition BD’s new fluorescent dyes, the Brilliant dye series, are making it possible to expand your panels into the 20+ color sphere.
For more information, help with project planning and training on the new instruments, please contact the FCCF at Montebello (flowcytometri@rr-research.no).
Links:
Flow Cytometry Core Facility home page
Some highlighted publications on high parameter cytometry:
https://www.nature.com/articles/s41590-018-0294-9
https://www.nature.com/articles/srep36641
https://www.sciencedirect.com/science/article/pii/S0092867419302673?via%3Dihub
https://www.sciencedirect.com/science/article/pii/S2405471217305434?via%3Dihub
https://onlinelibrary.wiley.com/doi/full/10.1002/cyto.a.23850
https://onlinelibrary.wiley.com/doi/full/10.1002/cyto.a.23853
