New insights into the molecular mechanism underlying a human developmental disorder published in Cell Reports

In a recent publication in the journal Cell Reports, Kari-Anne M Frikstad, in team with six international collaborators and her co-workers in Sebastian Patzke's project group at the Institute for Cancer Research, provides new insights on how cells build a functional antenna for sensation of a key developmental signaling pathway required for normal brain development.
The primary cilium can be considered as a confined cellular antenna on the cell surface, specialized for the sensation of developmental signaling pathways that are key for the implementation and maintenance of the body plan. This includes the Hedgehog (Hh) signaling pathway, which also is aberrantly exploited by various cancer forms for promotion of growth. CEP104 and CSPP1 belong to a group of 35 cilia-associated genes for which deleterious mutations have been described to cause Joubert syndrome (JBTS), a developmental disorder primarily defined by a characteristically underdeveloped mid- and/or hindbrain. Almost all affected JBTS-proteins are exclusively connected to the transition zone (TZ) at the base of the primary cilium and the regulation of Hh-signaling. CEP104 was an exception to this understanding, by being localized to the daughter centriole and the ciliary tip, but not the mother centriole or the TZ of the cilium/basal body entity, and by having an undetermined role in Hh-signaling or interaction with other JBTS-proteins.
Frikstad et al.now shows that loss of cep104 in zebrafish leads to shortened cilia in the Kupffer's Vesicle and to defective cranial nerve development (contribution from John Sayer's lab, Newcastle University). In human cells, intra-ciliary interaction of CEP104 and CSPP1 at the tip of the primary cilium was found to regulate cilia length and Hh-signaling competence. Impaired signaling was linked to reduced capability to localize the G-protein coupled receptor SMO to the cilium in response to pathway stimulation, which is an essential step in the signaling cascade. Hence, abrogation of this process is indicated as the underlying molecular defect in JBTS resulting from CEP104 mutations. More generally, the gained molecular insights shed new light on the enigmatic tip region of the primary cilium and may prove valuable for development of future strategies to treat tumors driven by ciliary signaling. Furthermore and in contrast to CEP104, CSPP1 proteins interact with microtubules of the cilium and the mitotic spindle apparatus. CSPP1 over-expression is observed in cancers of different histologies, and is promoting spindle assembly and chromosome congression failure in cell line models. Hence, the study adds another brick to the puzzle of understanding how cells spatially- and temporally-defined re-organize their microtubule cytoskeleton.
The project was realized with help of generous funding by Kreftforeningen.
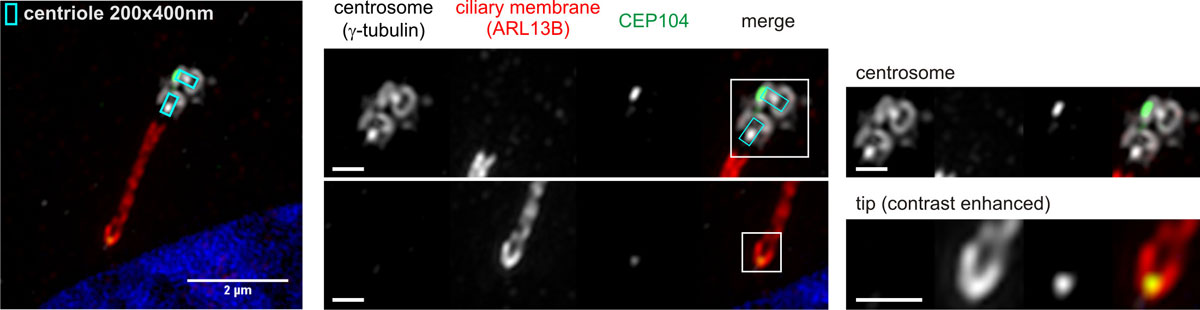
3D-Superresolution microscopy of the primary cilium expressed on the surface of an hTERT-RPE1 cell stably expressing mNeonGreen-tagged CEP104 showing localization of CEP104 to the distal end of the daughter centriole and the cilia membrane enclosed tip region of the primary cilium. Scale bars in close-up views: 500 nm. (click here to open figure in large format)
Links:
A CEP104-CSPP1 Complex Is Required for Formation of Primary Cilia Competent in Hedgehog Signaling
Kari-Anne M. Frikstad, Elisa Molinari, Marianne Thoresen, Simon A. Ramsbottom, Frances Hughes, Stef J.F. Letteboer, Sania Gilani, Kay O. Schink, Trond Stokke, Stefan Geimer, Lotte B. Pedersen, Rachel H. Giles, Anna Akhmanova, Ronald Roepman, John A. Sayer, Sebastian Patzke
https://doi.org/10.1016/j.celrep.2019.07.025
Home page of Sebastian Patzke's project group - Centrosome and Cell Division Cycle
