New findings on the inhibition of growth factor signaling: Paper from Wiedlocha group published in "Science Signaling"

An article from Antoni Wiedlocha's group at the Department of Biochemistry at the Institute for Cancer Research, entitled "ERK-Mediated Phosphorylation of Fibroblast Growth Factor Receptor 1 on Ser777 Inhibits Signaling" is published in the February 12th issuue of the prestigious journal "Science signalling" (journal impact factor 7.5).
This work demonstrates a previously uncharacterized ERK1/2-mediated a negative feedback mechanism for the control of FGFR1 protecting cells from excessive FGFR1 signaling.
A summary of the findings in the article, from the authors:
Fibroblast growth factor 1 (FGF1) controls cellular activities through the activation of specific cell-surface FGF receptors (FGFRs). Trans-phosphorylation of tyrosine residues in the kinase domain of FGFRs leads to activation of intracellular signaling cascades, including those mediated by mitogen-activated protein kinases (MAPKs). In addition to the kinase domain, FGFRs contain a serine-rich C-terminal tail of unknown function. Here, we identified a previously uncharacterized regulatory mechanism of FGFR signaling based on direct phosphorylation of a specific serine residue (Ser777) in the C-terminal region of FGFR1 by the MAPKs extracellular signal–regulated kinase 1 (ERK1) and ERK2. This serine phosphorylation event substantially reduced the extent of tyrosine phosphorylation in the kinase domain of the receptor. Prevention of the phosphorylation of FGFR1 Ser777 or mutation of Ser777 to alanine resulted in enhanced receptor tyrosine phosphorylation, as well as increased cell proliferation, cell migration, and axonal growth, whereas a phosphomimetic mutation at Ser777 led to reduced FGFR1 signaling. FGFR-independent activation of MAPKs also resulted in phosphorylation of FGFR1 Ser777, thereby enabling crosstalk regulation of FGFR activity by other signaling receptors. Our data reveal a negative feedback mechanism that controls FGF signaling and thereby protects the cell from excessive activation of FGFR.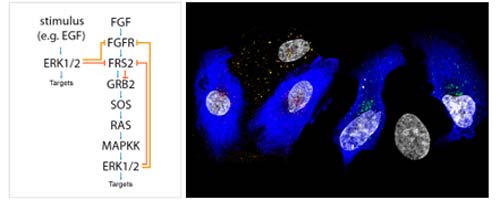
Links
M. Zakrzewska, E. M. Haugsten, B. Nadratowska-Wesolowska, A. Oppelt, B. Hausott, Y. Jin, J. Otlewski, J. Wesche, A. Wiedlocha
ERK-Mediated Phosphorylation of Fibroblast Growth Factor Receptor 1 on Ser777 Inhibits Signaling.
Sci. Signal. 12 Feb2013
Home page of Antoni Wiedlocha's group:
Protein internalisation and signaling - Nuclear translocation and intracrine signaling of FGFs
Centre for Cancer Biomedicine, UiO
