Findings from Ola Myklebost's group published in Cancer

This is the most extensive such study to date, and showed that although the detailed patterns of genomic aberrations are very similar between the donor samples and early transplants, clear differences can be observed. However, these are scattered all over the genome, and most likely are due to the heterogeneity in the donor tissues. Interestingly, in most cases the changes were towards more normal genomes, i.e. loss of aberrations, possibly due to reduced cellular heterogeneity caused by clonal simplification.
On the other hand, when changes that occur during serial transplantation in mice were scored, some significantly recurrent changes were observed, all in osteosarcomas, which are known for their high genomic instability. Aberrations that increased were of the same type as observed also in patient samples, and most likely were due to normal tumour progression. On the other hand, aberrations that disappeared contained genes that may be involved in chemotherapy response and immune evasion, and it seems likely that these could have appeared due to selection in the patient by chemotherapy and the immune response to the tumour, and were lost because these selection pressures were no longer present in the mice.
In conclusion, the xenografts are good preclinical models, resembling their tumours of origin well. The fluctuation of copy number aberrations reflects the natural heterogeneity in tumours, and thus may not be a significant disadvantage. The study also confirms the well-known caveats when using models in immune-deficient mice for studies of cancer-stroma interactions and immune-related mechanisms.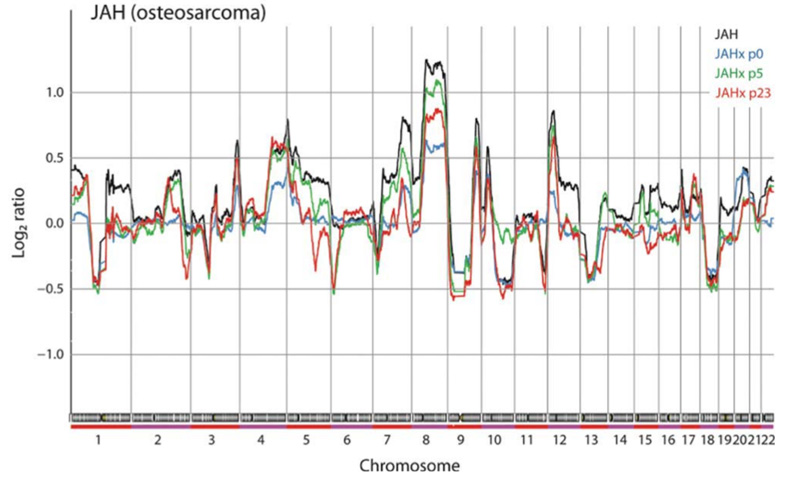
Links
Preclinical xenograft models of human sarcoma show nonrandom loss of aberrations.
Kresse SH, Meza-Zepeda LA, Machado I, Llombart-Bosch A, Myklebost O.
Cancer. 2011
European Network of Excellence Eurobonet
Cancer (journal published on behalf of the American Cancer Society)
Home page of Ola Myklebost's group
