Findings regarding a novel non-genomic tamoxifen cytotoxic action published in ACS Chemical Biology

First author
Theodossis (Theo) Theodossiou at the Department of Radiation Biology, in collaboration with two other departments at ICR and Department of Cellular Therapy (Authors: Theodossis Theodossiou, Sébastien Wälchli, Catherine Elisabeth Olsen, Ellen Skarpen and Kristian Berg), have published new research in ACS Chemical Biology regarding a novel non-genomic tamoxifen cytotoxic action. The results describe an alternative non-genomic utilization of tamoxifen that may be of clinical interest and describes also the mechanistic basis for this treatment effect. This non-genomic action could be of particular interest for estrogen receptor negative (or triple negative) cancers depending on their metabolic/redox physiology.
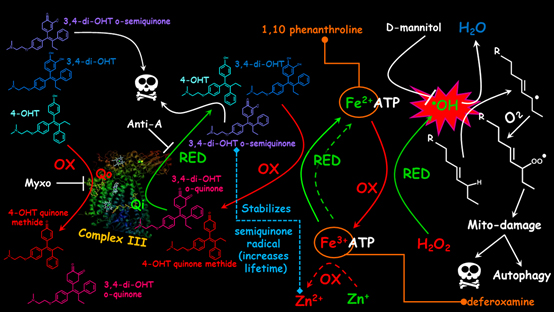
In this study a high single tamoxifen dose was used for treatment of breast cancer cells. It was found that tamoxifen has a better cytotoxic action in Pasteur (higher respiration) cells than in Warburg (glycolytic cells) cells. The cytotoxic action is due to a) lipid peroxidation caused by reduced tamoxifen quinoid species or the tamoxifen metabolite 4-hydroxytamoxifen which can provide Fenton-reactive Fe(II) or b) semiquinones of the tamoxifen quinoid species which are produced (are more stable) at the quinonereducing centre of the mitochondrial electron transport chain complex III, also leading to mitochondrial DNA damage. These findings suggest that tamoxifen could be utilized differently than in current clinical practice for respiratory and/or estrogen receptor negative cancers, i.e. instead of administrating a daily low tamoxifen dose the patients may be treated once or a limited number of times with high tamoxifen boluses, preferably in combination with a selective inhibition of the quinoloxidizing centre of complex III. The results also indicate that high dose tamoxifen administration should be avoided in cases of iron overload diseases such as β-thalassemia, hemochromatosis and porphyria cutanea tarda, as it could result to hepatotoxic conditions like severe liver siderosis (iron overload), non-alcoholic steatohepatitis (NASH), even cirrhosis and liver cancer. Preclinical evaluation of the single bolus high dose tamoxifen regimen is currently ongoing.
 |
Links:
Deciphering the non-genomic, mitochondrial toxicity of tamoxifens as determined by cell metabolism and redox activity.
Theodossiou TA, Wälchli S, Olsen CE, Skarpen E, Berg K.
ACS Chem Biol. 2015 Nov 16.
The PCI web site (home page of Kristian Berg's group)
