Angela Oppelt and colleagues identify novel lipid regulator of cell migration

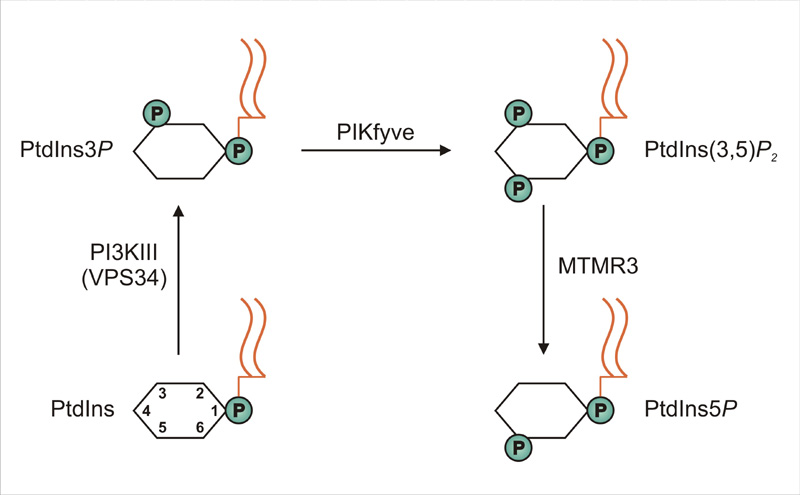
Because metastasis involves migration of cancer cells, there is great interest in cancer biology to identify mechanisms that regulate cell migration. In a recent issue of EMBO Reports (published on-line 16 Nov), PhD student Angela Oppelt (photo) and her colleagues in Jørgen Wesche's project group at the Institute for Cancer Research present a novel regulator of cell migration. The novel regulator is a lipid, PI5P (phosphatidylinositol 5-phosphate), which is generated from the common phospholipid, phosphatidylinositol, through a 3-step reaction catalyzed by the consecutive actions of the enzymes VPS34, PIKfyve and MTMR3.
The paper has already attracted considerable attention, and it is now (Dec 3rd) ranked as number one on the top-ten list of downloaded papers at EMBO Reports.
Interestingly, Oppelt and co-workers identified all these enzymes as regulators of cell migration, and when their function is impaired, cellS migrate at a slower rate. Conversely, if cellular levels of PI5P are artificially enhanced, cell migration is speeded up. The authors also showed that fibroblast growth factor, which is known to stimulate cell migration, causes a significant increase in cellular PI5P levels.
What is the role of PI5P in cell migration? Oppelt and co-workers found evidence that this lipid controls (unknown) proteins that promote remodelling of actin filaments, a prerequisite for cell migration.
Given the interest for targeting cell migration in anti-metastatic therapy, the paper from Oppelt and co-workers has already attracted considerable attention, and it is already ranked as number one on the top-ten list of downloaded papers at EMBO Reports.
Links:
Production of phosphatidylinositol 5-phosphate via PIKfyve and MTMR3 regulates cell migration.
Oppelt A, Lobert VH, Haglund K, Mackey AM, Rameh LE, Liestøl K, Oliver Schink K, Marie Pedersen N, Wenzel EM, Haugsten EM, Brech A, Erik Rusten T, Stenmark H, Wesche J.
EMBO Rep. 2012 Nov 16. doi: 10.1038/embor.2012.183. [Epub ahead of print] Download PDF
Top-ten list of downloaded papers at EMBO Reports
Home page of Jørgen Wesche's project group - Cancer cell migration and metastasis