Viola Lobert discovers functional importance of integrin degradation. Commented on the Cell Migration Gateway.

In the July 2010 issue of Developmental Cell (impact factor 13.36), PhD student Viola Lobert and her co-workers in Harald Stenmark´s group at the Centre for Cancer Biomedicine, Institute for Cancer Research, demonstrate an unexpected importance of integrin degradation in cell migration.
The findings have recently attracted considerable attention. The Cell Migration Gateway - which represents a unique collaboration between The Cell Migration Consortium (CMC) and Nature Publishing Group (NPG) and is designed to facilitate navigation through the complex world of cell migration research - presents a comprehensive referral to the article in its August update.
Integrins are heterodimeric membrane proteins that form contacts between cytosolic cytoskeletal components and the extracellular matrix. Because of their involvement in cell migration, which is crucial for tumour invasion, Lobert and co-workers have been studied the regulation of integrin molecules in migrating cells. It is well known that endocytosis and subsequent recycling of the fibronectin receptor, alpha5beta1 integrin, is crucial for cell migration through controlling the correct formation of adhesion site at the leading lamellopodium of migrating cells. Through microscopic studies of migrating fibroblasts, Lobert and colleagues made the unanticipated observation that a substantial fraction of integrin molecules were found in multivesicular endosomes and late endosomes, organelles normally associated with degradative functions. The authors went on to show that a fraction of alpha5beta1 integrin is indeed degraded in lysosomes, and that the sorting to these organelles is mediated by fibronectin-induced ubiquitination of the alpha5 chain, followed by recognition and sorting of the ubiquitinated integrins by the endosomal sorting complex required for transport (ESCRT) machinery in endosomal membranes. Importantly, interference with this sorting inhibits cell migration, revealing that lysosomal sorting of alpha5beta1 integrin is crucial for proper cell migration. Lobert and colleagues conclude that it is important for the migrating cell to degrade fibronectin-bound integrin molecules that are internalised, since recycling of such molecules would otherwise cause dysfunctional adhesion sites (see Figure).
Lobert comments that "we were surprised to make these observations, since everyone in the integrin field has been assuming that essentially all endocytosed integrin molecules are recycled. The importance of lysosomal trafficking of integrins in fibroblast migration raises the interesting question whether such trafficking could also be important in tumour cell migration, and whether this may be targeted in cancer therapy."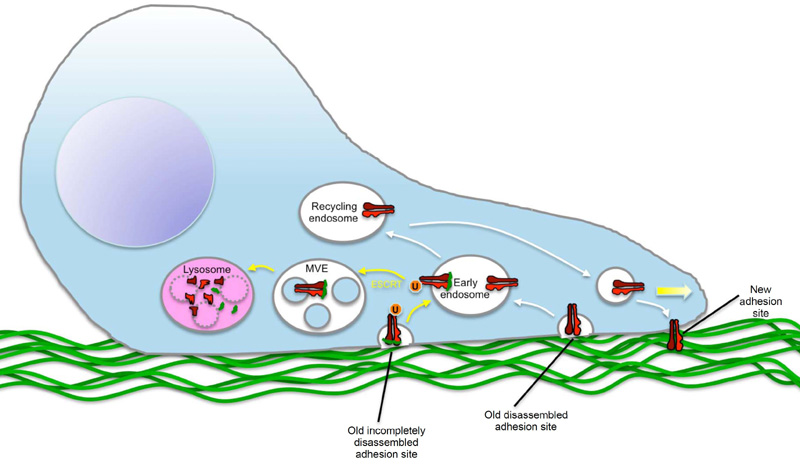
Links:
Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes.
Lobert VH, Brech A, Pedersen NM, Wesche J, Oppelt A, Malerød L, Stenmark H.
Dev Cell. 2010 Jul 20;19(1):148-59.
Feature article from the August update of the "Cell Migration Gateway":
Cell migration: ESCRTed by integrin degradation
