Research & Methods
RESEARCH PROJECTS
CLL-OUTCOME:
Chronic lymphocytic leukemia: Improving survival and quality of life
Chronic lymphocytic leukemia (CLL) is the most common form of leukemia in Europe and it remains incurable. Targeted therapies have revolutionized the treatment of CLL. However, many patients develop resistance, have severe side effects, or relapse during treatment. To prevent ineffective treatment and reduce toxic effects, there is an unmet clinical need for tailoring optimal therapy to each patient.
In CLL-OUTCOME, we aim to improve survival and health related quality of life (hrQoL) for patients with CLL; specifically, to increase treatment efficacy of targeted therapies and to reduce adverse events. The consortium has previously identified biomarkers for predicting clinical outcomes and developed a novel machine learning-based prognostic model based on these biomarkers as a clinical decision support tool in the ERA PerMed project CLL-CLUE (see below). Here, we will validate this prognostic model in a feasibility study using samples and data from clinical studies. This will enable us to optimize the model input and individual patient outcomes for hrQoL and treatment efficacy in preparation for a prospective clinical trial. To facilitate implementation of the clinical decision support tool in the healthcare system, we will develop health economic models to assess the prognostic model retrospectively and prospectively, with emphasis on involving key stakeholders, including patient representatives and pharmaceutical companies with drug development for CLL, both in product development and when deciding trial endpoints.
The CLL-OUTCOME consortium:
Coordinator:
Sigrid S. Skånland (Oslo University Hospital, Norway)
Partners:
Tero Aittokallio (University of Helsinki, Finland)
Barbara Eichhorst (University Hospital of Cologne, Germany)
Carsten Utoft Niemann (Copenhagen University Hospital, Denmark)
Thorsten Zenz (University of Zurich, Switzerland)
Carin Uyl-de Groot (Erasmus University Rotterdam, the Netherlands)
Olav Ljøsne (The Norwegian Blood Cancer Association, Norway)

CLL-CLUE: Tailoring the targeted treatment of chronic lymphocytic leukemia
Chronic lymphocytic leukemia (CLL) is the most common form of leukemia in Europe and it remains incurable. Targeted therapies have revolutionized the treatment of CLL. However, many patients develop resistance, have severe side effects or relapse during treatment. There is an unmet medical need for tailoring optimal therapy for each patient in order to prevent ineffective treatment and toxic side effects. The CLL-CLUE project will identify multi-omics biomarker panels and implement artificial intelligence-based clinical decision support systems to guide personalised treatment decisions. We expect that this will lead to significantly increased treatment efficacy, individualisation of therapy and reduced drug use and side effects. In addition, reduced consumption of drugs and cost-effective outcomes will lower financial stress that the health care providers and patients experience.
The CLL-CLUE consortium:
Coordinator:
Sigrid S. Skånland (Oslo University Hospital, Norway)
Partners:
Tero Aittokallio (University of Helsinki, Finland)
Barbara Eichhorst (University Hospital of Cologne, Germany)
Carsten Utoft Niemann (Copenhagen University Hospital, Denmark)
Thorsten Zenz (University of Zurich, Switzerland)
László Zsombor Lorenzovici (Syreon Research Romania SRL, Romania)
IMPRESS-Norway: A chronic lymphocytic leukemia cohort
IMPRESS-Norway (NCT04817956) is a prospective, non-randomized clinical trial evaluating efficacy of commercially available, anti-cancer drugs prescribed for patients with advanced cancer diagnosed with potentially actionable alterations as revealed by molecular diagnostics. We have opened a cohort for patients with relapsed/refractory chronic lymphocytic leukemia (CLL) in this trial. These patients will receive treatment with a proteasome inhibitor following a positive drug sensitivity test on their cancer cells. The method is developed in our lab (Hermansen et al, Cell Death Discov, 2023). This is the first cohort to receive treatment guided by a functional biomarker in IMPRESS-Norway, and the first time drug sensitivity testing is used to guide treament of CLL patients in a clinical trial. We previously showed that this approach can successfully guide treatement of relapsed/refractory CLL (Skånland et al, Haematologica, 2022).
METHODS
Drug sensitivity screening
The principle of drug sensitivity testing is to expose cancer cells to a library of different drugs and measure its effects on cell viability. We have optimized and standardized methods to culture and screen primary cancer cells against a customized drug library (Ayuda-Durán et al, Cell Death Discov, 2023). The Figure (below) shows the common principles of drug sensitivity protocols for hematologic cancers. Cell isolation and pre-culture (left panels) are specific to primary cells and are assay dependent. Primary cells or cell lines are incubated with the drug library for 3 days. Cell viability is then assessed, and the data are analyzed and presented. The read-outs may provide insight into tumor biology, identify or confirm treatment resistance and sensitivity in real time, and ultimately guide clinical decision-making.
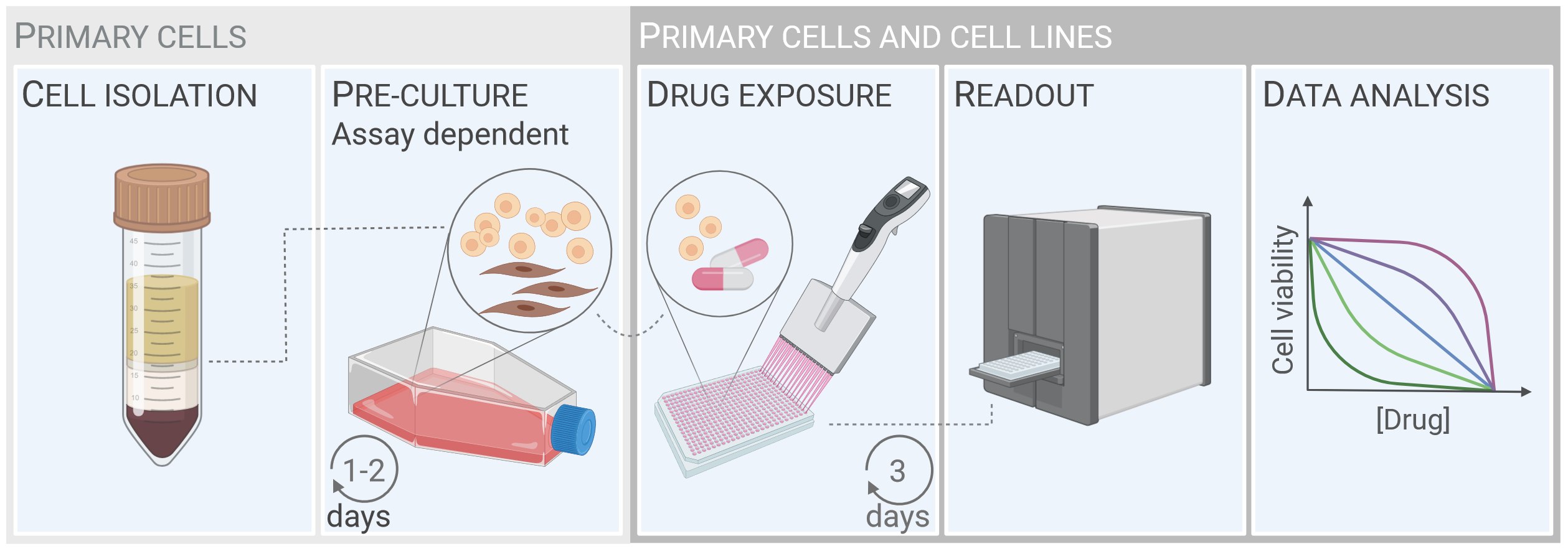
The workflow for drug sensitivity screens on patient cells - from cell isolation from blood samples to data analysis.
The figure is from Ayuda-Durán et al, 2023
Immune phenotyping with (phospho)protein profiling
The liquid nature of peripheral blood makes this organ uniquely suited for single-cell studies by flow cytometry. (Phospho)protein profiles detected by flow cytometry analyses have been shown to correlate with ex vivo drug sensitivity and to predict treatment outcomes in hematologic cancers, demonstrating that this method is suitable for pre-clinical studies. We have developed a flow cytometry protocol that combines multi-parameter immune phenotyping with single-cell (phospho)protein profiling (Figure, left). The protocol makes use of fluorescent cell barcoding (Figure, right), which means that multiple cell samples, either collected from different donors or exposed to different treatment conditions, can be combined and analyzed as one experiment. This increases the throughput of the experiment, reduces variability between samples, and lowers experimental costs. The read-outs may provide biological insight to cancer pathogenesis, identify novel drug targets, and ultimately serve as a biomarker to guide clinical decisions.
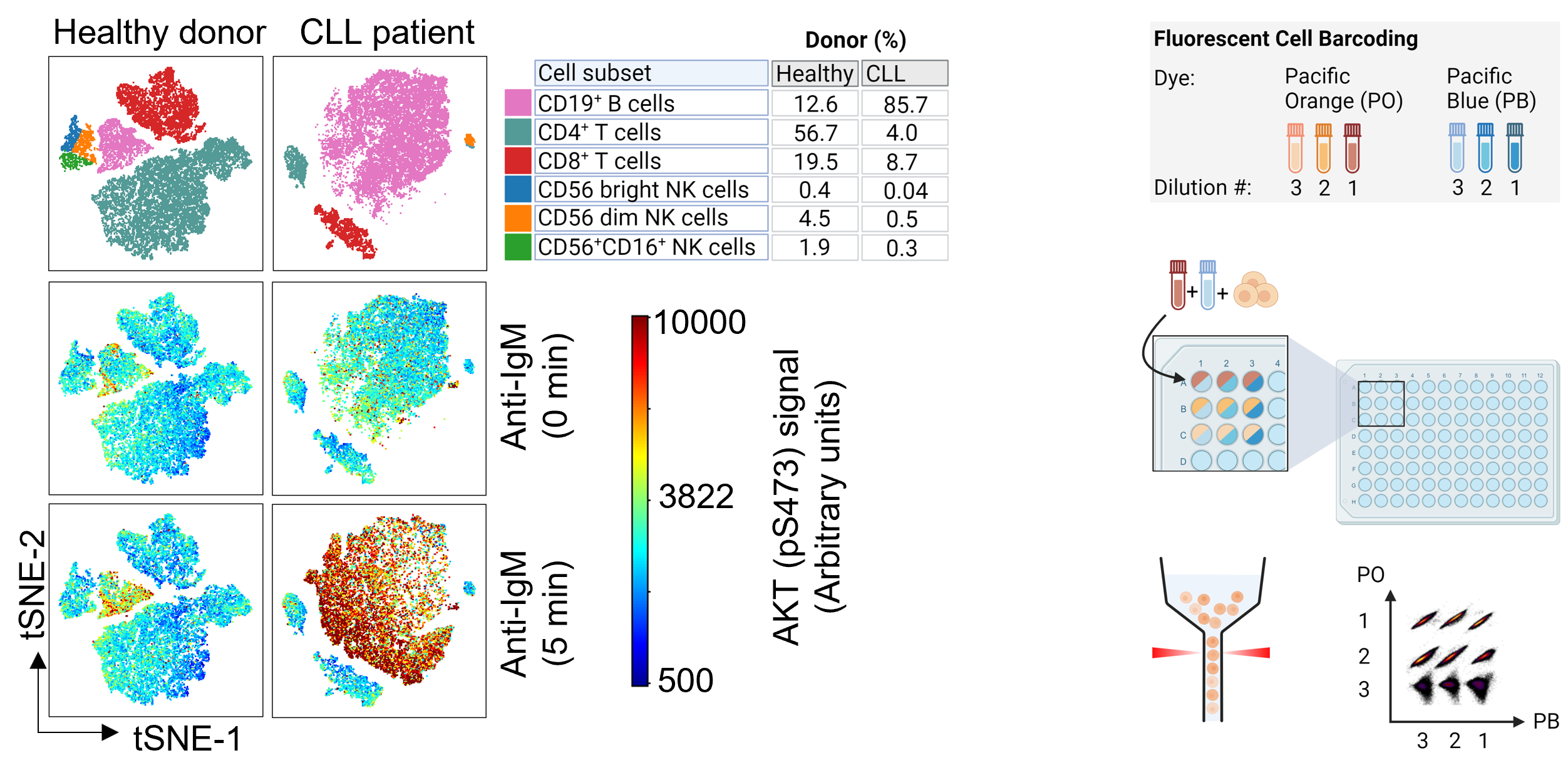
Representative immunophenotypes of samples from a healthy donor and a CLL patient (left). Immunophenotyping can be combined with fluorescent cell barcoding (right). The figure is adapted from Hermansen et al, NPJ Precision Oncology, 2024.
The video below shows the step-by-step protocol for (phospho)protein profiling with fluorescent cell barcoding:

