Selbos project group - Light-controlled delivery of cancer immunotherapeutics Research projects
A major barrier to cancer therapy is sequestration of therapeutic agents in endosomes and lysosomes, which may include peptides/proteins, RNA/DNA, nanoparticles and weak chemotherapeutic bases. To solve this problem, photochemical internalisation (PCI) was developed as a minimal invasive drug delivery technology for light-controlled cytosolic release of molecules/particles entrapped in endosomes and lysosomes. PCI has been demonstrated in >80 different cancer cell lines and in >10 different animal models (Review: Selbo PK et al., J Control Release, 2010).
PCI has been shown to be safe and efficient in several clinical studies. The first-in-human clinical study was based on PCI of bleomycin to treat different solid cutaneous or subcutaneous malignancies and published in Lancet Oncology (Sultan et al, 2016). Preclinical work in our project group has contributed with data important for the following two clinical trials:
1) PCI of HPV peptide-based vaccine antigens in healthy volunteers published in Frontiers of Immunology (Otterhaug et al, 2021).
2) PCI of gemcitabin for the treatment of inoperable CCA is currently ongoing in the pivotal Phase II multi-centre RELEASE study in Europe, USA and Asia with the ClinicalTrials.gov Identifier: NCT04099888.
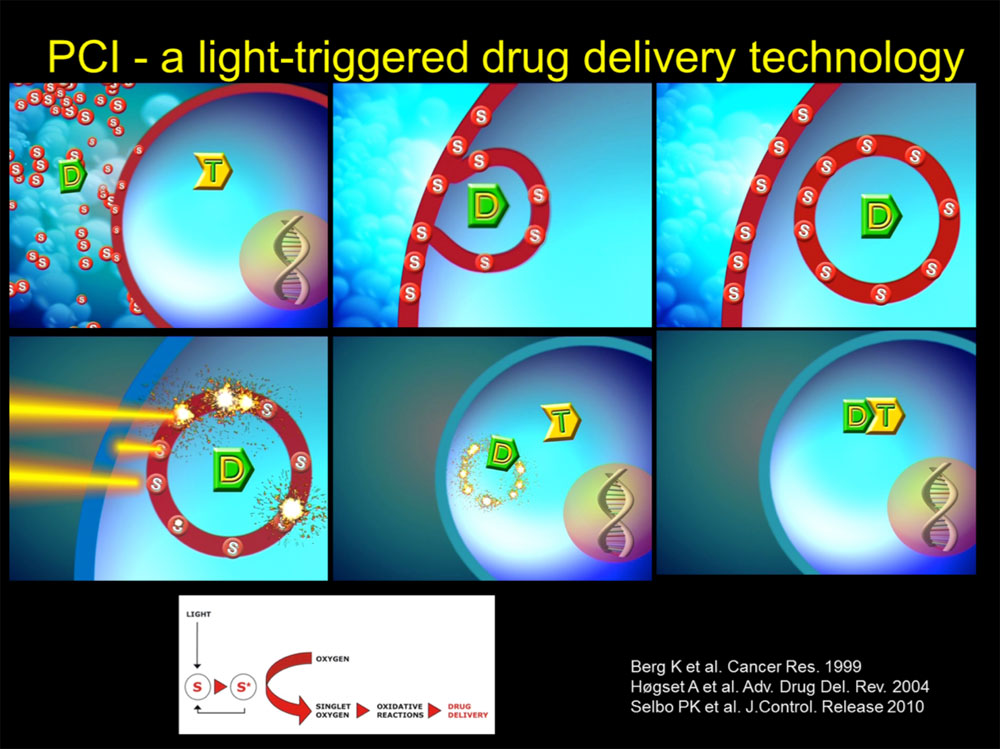
Figure 1. Photochemical internalisation (PCI) is an intracellular drug delivery technology that induces endosomal escape. PCI is based on the combination of the endosome and lysosome-targeting photosensitiser (S) fimaporfin, also known as TPCS2a, that is given together with a drug (D), e.g. peptide/protein, RNA/DNA, nanoparticle or weak base drug, which also accumulates in the same endocytic vesicles. Therapeutic macromolecular drugs that do not penetrate the plasmamembrane are transported inside the cancer cells by endocytosis and accumulate subsequently in endocytic vesicles. Weak base drugs (e.g. chemo or TKIs) accumulate in the acidic lumen of endosomes/lysosomes due to protonation. The amphiphilic PCI photosensitiser becomes incorporated in the plasmamembrane and is taken up into cells by endocytosis and co-localises with the drug of interest. Spatiotemporal controlled light excitation of the photosensitiser results in energy transfer to oxygen and generation of reactive oxygen species (ROS). Photochemically induced ROS facilitates lipid peroxidation and leaky endosomal and lysosomal membranes causing cytosolic release of the sequestered drug.
Project I: PCI of immunotoxins targeting cancer stem cells
Despite progress in radio-, chemo- and targeting-based therapy of cancer, treatment resistance still remains a major problem for the majority of patients with aggressive tumours. Even though cancer therapy results in complete or partial response, tumour re-grow take place in too many patients after end of treatment. Cancer stem cells (CSCs) are notoriously resistant to conventional cancer therapies and suggested to be responsible for the tumour recurrence after therapy. Due to the CSCs ability to both self-renew and differentiate they are capable to drive and maintain tumour development and heterogeneity. Thus, there is a high need for novel technologies that target and kill CSCs.
We have demonstrated that PCI-based photosensitizers are not substrates of efflux pumps of the ATP-binding cassette (ABC) transporters such as ABCB1 (also known as MDR1 or P-gp) and ABCG2 that are often overexpressed on multi-drug resistance cancer cells, in particular CSCs. By use of the PCI method, we have shown that it is possible to target and kill CSCs by light-triggered activation of immunotoxins (both chemical conjugates and recombinant fusion toxins) targeting CD133, CD44, EpCAM and CSPG4.
Project II: PCI of cancer vaccine antigens
Entrapment and degradation of vaccine antigens in lysosomes is a major obstacle for efficient therapeutic cancer vaccines. In an industry-academic collaboration with PCI Biotech (and University Hospital Zürich, NTNU and Leiden University Medical Center), we have demonstrated preclinically and clinically that the PCI method enhance the efficacy of peptide and protein-based vaccine antigens. By using the PCI method we are able to induce endosomal escape of vaccine antigens in antigen-presenting cells (APC). The PCI-induced cytosolic delivery is followed by strong enhancement of MHC class I presentation of the vaccine antigen. This way of enhanced cross-presentation leads to strongly improved activation of antigen-specific CD8+ cytotoxic T-cell lymphocytes (killer T-cells) that are crucial for the direct kill of tumour cells.
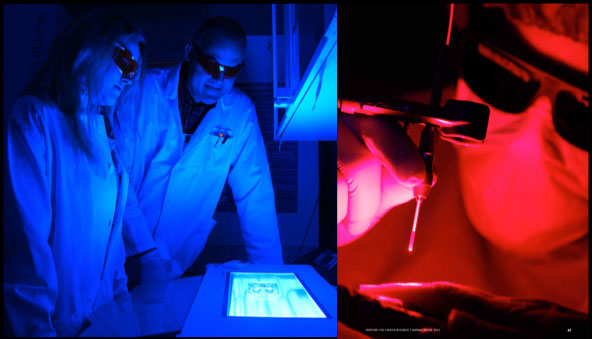
Figure 2. Both blue and red light can be used for PCI. The use of different wave-lengths of the light is possible because the photosensitizer has several absorption peaks including a major one in the blue region (λmax ≈ 420 nm) and one in the deep red (λmax = 652 nm). Left image, we are using blue light for activation of the PCI photosensitizer in cancer cell cultures (Photo by Arnfinn Christensen, forskning.no). Right image, red light penetrates deeper into tissues than blue light and is therefore used when treating e.g. tumours. In this photo the PI is shining red laser light on the index finger of a healthy volunteer (Photo by Terje Heiestad).
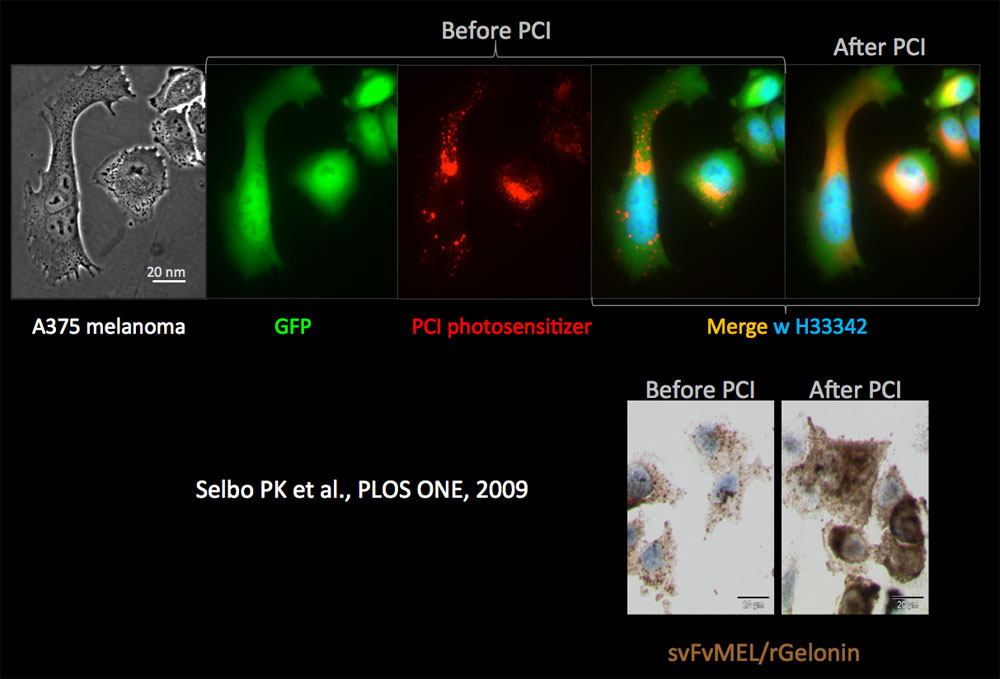
Figure 3. PCI-mediated cytosolic delivery of photosensitizer and CSPG4-tageting recombinant fusion toxin (immunotoxin) scFvMEL/rGel. Upper four panels, GFP expressing (green) A375 melanoma cells with PCI photosensitizer (red) and nucleus stained with Hoechst 33342 (blue). Before light exposure, the shape of the fluorescence signal of the photosensitiser is granular (puncta). After light activation the photosensitiser fluorescence puncta disappears and becomes diffuse, indicating cytosolic delivery (PCI). Lower right panels, immunocytochemical detection of the ribosomal inactivating protein rGel (recombinant gelonin), which is the active drug moiety of the fusion toxin (scFvMEL/rGel) before and after PCI in the A375-GFP cells. The puncta signals of rGel resemble the shape of endosomes and lysosomes, while the diffuse rGel signal indicate cytosolic delivery by PCI.
