Journal of Cell Biology publication: BEACH domain proteins as new guides in transmembrane cargo sorting

first author
Around one-fourth of all eucaryotic proteins, including cell surface receptors, are transmembrane proteins transported to their final destination along the lipid bilayer of the cellular endomembrane system. The protein sorting mechanisms that guide this transport and the pathways taken by transmembrane proteins are still not clearly defined.
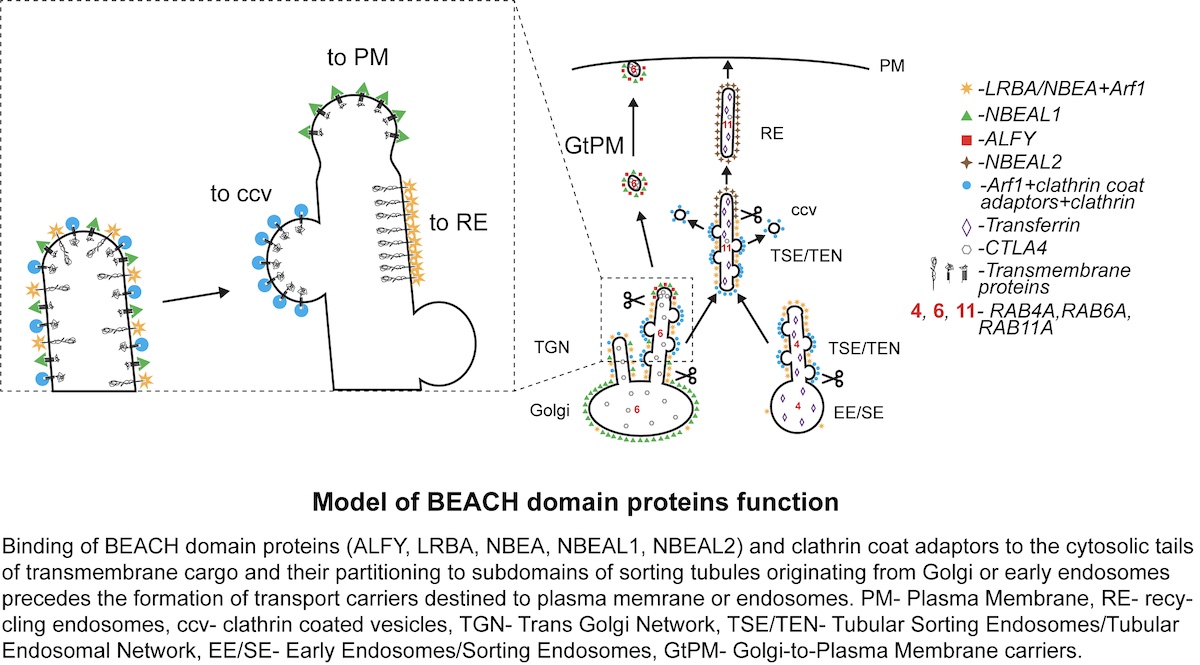
Now, researcher Serhiy Pankiv and co-workers in Anne Simonsen’s group identified the family of large BEACH domain-containing proteins (BDCPs) as sorting adaptors that, together with clathrin coat proteins, facilitate transport of transmembrane cargo to their destination. Using an extensive set of endomembrane markers, they mapped the subcellular localization of seven BDCPs. Intriguingly, five typical BDCPs localize to subdomains of highly dynamic sorting tubular compartments that receive cargo input from both secretory and endocytic recycling pathways and give rise to transport carriers destined for the plasma membrane or endosomes. Several BDCPs were found to bind to the cytosolic tail of the immune checkpoint receptor CTLA4 as well as other transmembrane cargos. The data support a model where BDCPs, together with clathrin coat adaptors, create a competitive molecular sieve that sorts transmembrane cargo either into clathrin-coated vesicles or BDCP-positive tubular carriers.
Links:
The Journal of Cell Biology article:
BEACH domain proteins as new transmembrane cargo sorting adaptors.
Pankiv S., Dahl AK, Aas A, Andersen RL, Brech A, Holland A, Singh S, Bindesbøll C, Simonsen A.
J Cell Biol (2024) 223 (12): e202408173. https://doi.org/10.1083/jcb.202408173
A commentary to the paper in JCB:
BEACH domain proteins in membrane trafficking and disease
Anne Simonsen’s group: Autophagy
