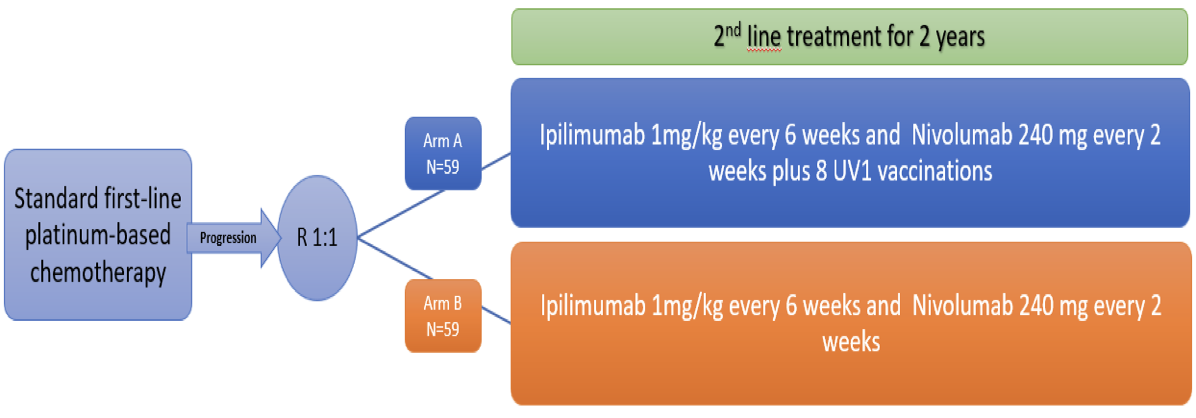
Malignant mesothelioma treated with immune checkpoint inhibitors with or without cancer vaccine (the NIPU-study) Study design

A total of 118 patients will be randomised into two study groups: ipilimumab and nivolumab alone or with UV1 vaccination. Treatment will be continued until disease progression, unacceptable toxicity for a maximum of 2 years. Because of the potential for pseudoprogression/tumour immune infiltration, this study will allow patients to remain on study treatment after apparent radiographic progression, provided the risk/benefit ratio is judged to be favourable.