IMPRESS-Norway Study design
IMPRESS-Norway is a combined basket- an umbrella trial where multiple targeted therapies are evaluated for multiple cancer types. Patients with the same cancer type and tumour profile or actionable molecular target are grouped into one cohort and treated with one drug or therapy.
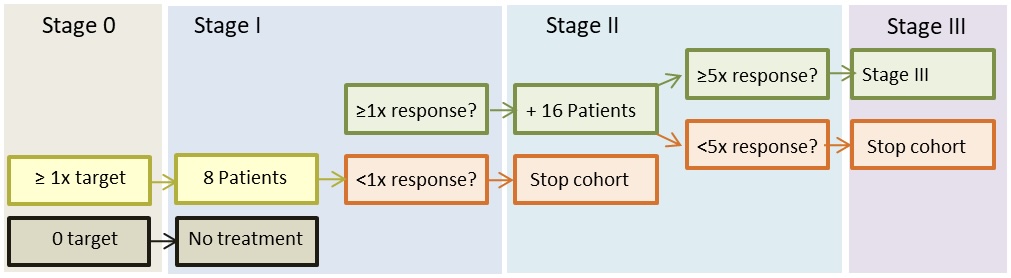
The stage I cohorts will have 8 patients. If ≥1 patient have treatment response after 16 weeks, 16 additional patients will be included in the stage II cohort. If ≥5 of the 24 patients show objective response after another 16 weeks, additional patients will be included in at stage III cohort and followed until disease progression.
Biologic material, including tumour tissue and blood samples are collected before treatment, during treatment and upon progression.
