COM-IT-2 Study design
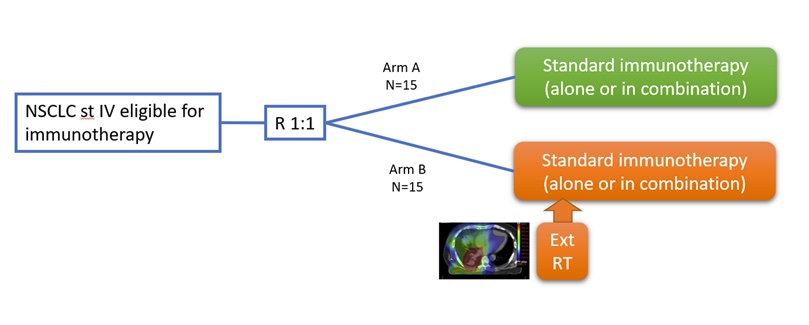
The COM-IT-2 trial is a translational study to identify biomarkers and optimize the combination of radiotherapy and immunotherapy using material from the COM-IT-2 trial and previous trials. The COM-IT-2 trial is a randomised phase 2 study comparing the efficacy and safety of systemic treatment with immunotherapy with or without extensive radiotherapy for patients with non-small cell lung cancer (NSCLC). A total of 30 patients will be included. Patients with stage IV NSCLC are eligible for inclusion at time of clinical indication of starting systemic treatment with immunotherapy. They will be randomized to standard systemic treatment or addition of extensive radiotherapy including as large parts of the tumour volume as possible. Identification of biomarkers will help identify patients in need of additional radiotherapy, and possibly other treatments. The clinical trial assesses toxicity and efficacy of the combined treatment. A definitive trial will be designed on the basis of the translational and clinical results.
This COM-IT-2 is a stepping stone to the creation of a larger, definitive trial of personalised immunoradiation including extensive radiotherapy to increase the clinical benefit of ICI treatment and by that increase survival of NSCLC. The trial will provide data on tolerance and feasibility of extensive radiotherapy necessary for a definitive trial. Translational analyses from analyses of biological material from this trial, combined with material from our previous trials, may provide biomarkers to personalise the immune-optimisation approach and thereby modify the design of the definitive trial and may introduce additional treatment arms. Increased survival with good quality of life is our long-term goal and combining strategies from oligometastatic radiotherapy trials and immunotherapy trials promise that this goal is within reach.