Testicular germ cell tumour
This project is currently run jointly between the Skotheim lab and Lothe lab at the Department of Molecular Oncology.
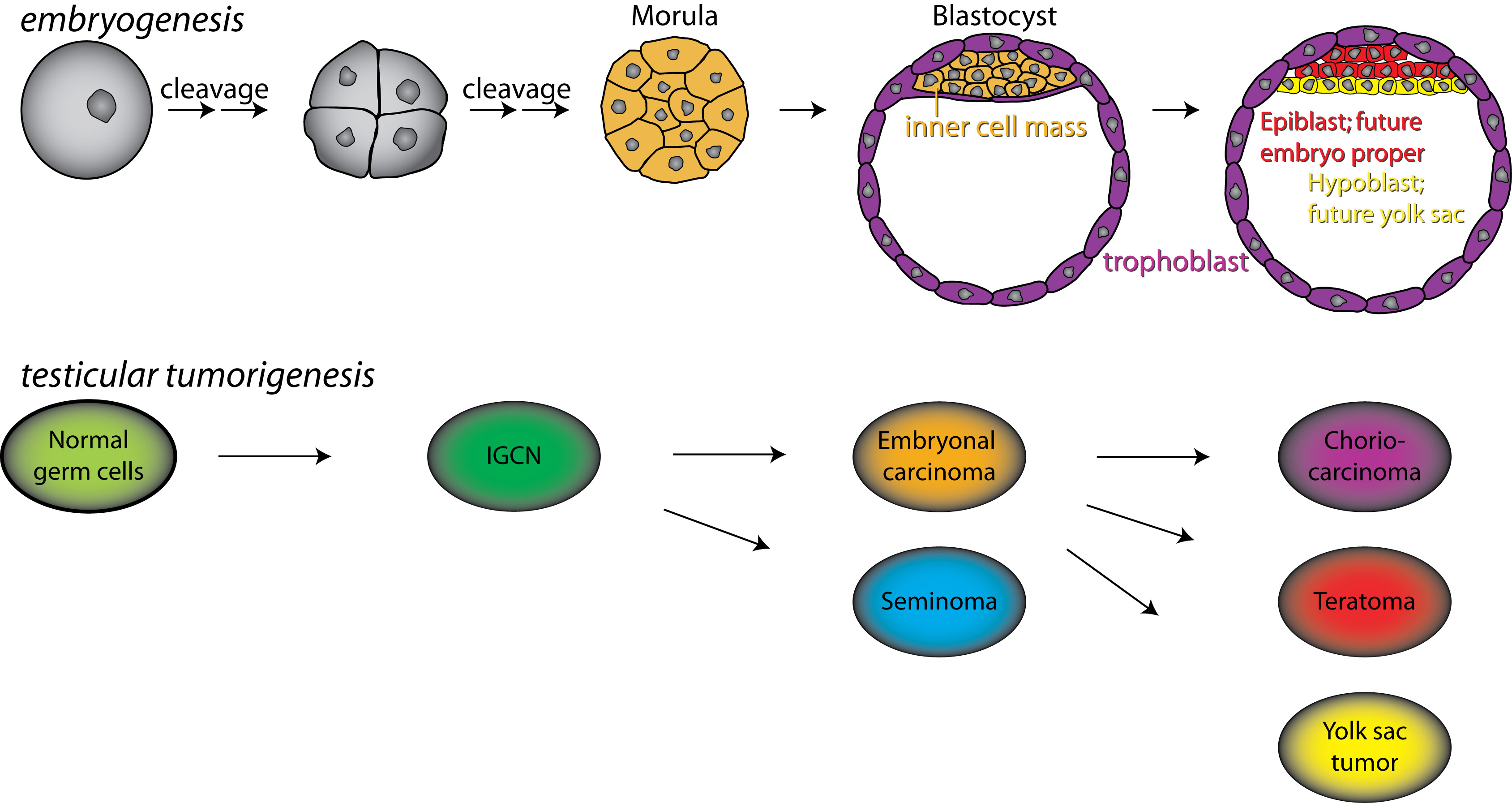
Testicular germ cell tumour (TGCT) is the most common type of cancer among young men (15 to 45 years of age). The incidence is increasing and in families, the relative risk is high. The tumors are either nonseminomas or seminomas or a combination. The non-seminoma group includes embryonal carcinomas, choriocarcinoma, teratoma and yolk sac tumors or combinations of these histological subtypes. The development of TGCT is a malignant caricature of the embryogenesis as illustrated.
Genetic risk for TGCT and long-term effects after chemotherapy
As partners in the Global testicular cancer consortium, TECAC, we seek to identify genetic risk markers for TGCT.
Selected publications
Grasso et al. & TECAC (2022). Association study between polymorphisms in DNA methylation-related genes and testicular germ cell tumor risk. Cancer Epidemiol. Biomarkers Prev. 31(9): 1769-79
Pluta et al. & TECAC (2021). Identification of 22 novel loci associated with susceptibility to testicular germ cell tumors. Nat. Commun. 12(1): 4487
Chung CC*, Kanetsky PA*, Wang Z*, Hildebrandt MAT*, Koster R*, Skotheim RI*, Kratz CP*, Turnbull C*, Cortessis VK*, [21 additional authors including Bakken AC, Kraggerud SM, Lothe RA], Chanock SJ, and Nathanson KL (2013). Meta-analysis identifies four new loci associated with testicular germ cell tumor. Nat. Genet. 45(6): 680-685 *Equal contribution between nine authors
Schumacher FR et al. & TECAC (2013). Testicular germ cell tumor susceptibility associated with the UCK2 locus on chromosome 1q23. Hum. Mol. Genet. 22(13): 2748-53
Kraggerud SM, Oldenburg J, Alnaes GI, Berg M, Kristensen VN, Fosså SD, and Lothe RA (2009). Functional glutathione S-transferase genotypes among testicular germ cell tumor survivors: associations with primary and post-chemotherapy tumor histology. Pharmacogenet. Genomics 19(10): 751-9
Kraggerud SM, Lee MP, Skotheim RI, Stenwig AE, Fosså SD, Feinberg AP, and Lothe RA (2003). Lack of parental origin specificity of altered alleles at 11p15 in testicular germ cell tumors. Cancer Genet. Cytogene. 147(1): 1-8
Skotheim RI, Kraggerud SM, Fosså SD, Stenwig AE, Gedde-Dahl Jr T, Danielsen HE, Jakobsen KS, and Lothe RA (2001). Familial/bilateral and sporadic testicular germ cell tumors show frequent genetic changes at loci with suggestive linkage evidence. Neoplasia 3(3): 196-203
Tumour stem cells and TGCT
Embryonal carcinoma (EC) cells, found in certain testicular germ cell tumours, represent a striking paradigm for malignant cells with stemness properties. Their pluripotent state allows them to self-renew, as well as differentiate, to give a disorganized array of cell and tissue types. EC cells are phenotypically similar to embryonic stem (ES) cells, which can be considered the non-malignant counterpart of EC cells. By comparing gene expression of EC and ES cells, we have gained insight into the role of stem cells in the development and progression of TGCT.
Selected publications:
Hoff AM*, Kraggerud SM*, Alagaratnam S*, Berg KCG, Johannessen B, Høland M, Nilsen G, Lingjærde OC, Andrews PW, Lothe RA#, and Skotheim RI# (2020). Frequent copy number gains of SLC2A3 and ETV1 in testicular embryonal carcinomas. Endocr. Relat. Cancer 27(9): 457-468 *#Equal contribution
Brabrand S*, Johannessen B*, Axcrona U, Kraggerud SM, Berg KG, Bakken AC, Bruun J, Fosså SD, Lothe RA, Lehne G, and Skotheim RI (2015). Exome sequencing of bilateral testicular germ cell tumors suggests independent development lineages. Neoplasia 17(2): 167-174 *Equal contribution
Alagaratnam S, Harrison NJ, Bakken AC, Hoff AM, Jones M, Sveen A, Moore H, Andrews PW, Lothe RA, and Skotheim RI (2013). Transforming pluripotency: an exon-level study of malignancy-specific transcripts in human embryonal carcinoma and embryonic stem cells. Stem Cells Dev. 22(7): 1136-1146
Goodmann M, Gashaw I, Eildermann K, Schweyer S, Bergmann M, Skotheim RI, and Behr R (2009). The pluripotency transcription factor Krüppel-like factor 4 is strongly expressed in intratubular germ cell neoplasia unclassified and seminoma. Mol. Hum. Reprod. 15(8): 479-88
Skotheim RI, Lind GE, Monni O, Nesland JM, Abeler VM, Fosså SD, Duale N, Brunborg G, Kallioniemi O-P, Andrews PW, and Lothe RA (2005). Differentiation of human embryonal carcinomas, in vitro and in vivo, reveals expression profiles relevant to normal development. Cancer Res. 65(13): 5588-5598
Molecular landscape of TGCT
Somatic changes at the genetic, epigenetic and expression level have been investigated in all histological subtypes.
Selected publications:
Zhao S*, Hoff AM*, and Skotheim RI (2020). ScaR - A tool for sensitive detection of known fusion transcripts: Establishing prevalence of fusions in testicular germ cell tumours. NAR Genomics and Bioinformatics 2(1): 1-12 *Equal contribution
Hoff AM, Alagaratnam S, Zhao S, Bruun J, Andrews PW, Lothe RA, and Skotheim RI (2016). Identification of novel fusion genes in testicular germ cell tumors. Cancer Res. 76(1): 108-116
Hofer MD, Browne TJ, He L, Skotheim RI, Lothe RA, and Rubin MA (2005). Identification of two molecular groups of seminomas by using expression and tissue microarrays. Clin. Cancer Res. 11(16): 5722-9
Skotheim RI, Abeler VM, Nesland JM, Fosså SD, Holm R, Wagner U, Flørenes VA, Aass N, Kallioniemi OP, and Lothe RA (2003). Candidate genes for testicular cancer evaluated by in situ protein expression analyses on tissue microarrays. Neoplasia 5(5): 397-404
Smith-Sørensen B, Lind GE, Skotheim RI, Fosså SD, Fodstad Ø, Stenwig AE, Jakobsen KS, and Lothe RA (2002). Frequent promoter hypermethylation of the O6-Methylguanine-DNA Methyltransferase (MGMT) gene in testicular cancer. Oncogene 21(57): 8878-84
Skotheim RI, Monni O, Mousses S, Fosså SD, Kallioniemi OP, Lothe RA, and Kallioniemi A (2002). New insights into testicular germ cell tumorigenesis from gene expression profiling. Cancer Res. 62(8): 2359-64
Kraggerud SM, Aman P, Holm R, Stenwig AE, Fosså SD, Nesland JM, and Lothe RA (2002). Alterations of the fragile histidine triad gene, FHIT, and its encoded products contribute to testicular germ cell tumorigenesis. Cancer Res. 62(2): 512-7
Reviews and editorials
Rajpert-De Meyts E and Skotheim RI (2018). Complex polygenic nature of testicular germ cell cancer suggests multifactorial aetiology. Eur. Urol. 73(6): 832-833
Kraggerud SM, Hoei-Hansen CE, Alagaratnam S, Skotheim RI, Abeler VM, Rajpert-De Meyts E, and Lothe RA (2013). Molecular characteristics of malignant ovarian germ cell tumors and comparison with testicular counterparts: implications for pathogenesis. Endocr. Rev. 34(3): 339-76
Alagaratnam S, Lind GE, Kraggerud SM, Lothe RA, and Skotheim RI (2011). The testicular germ cell tumour transcriptome. Int. J. Androl. 34(4 Pt 2): e133-50
Lind GE, Skotheim RI, and Lothe RA (2007). The epigenome of testicular germ cell tumors. APMIS 115(10): 1147-60
Skotheim RI and Lothe RA (2003). The testicular germ cell tumour genome. APMIS 111(1): 136-50