S100A4
 |
S100A4
This 11kDa protein belongs to the S100 family of calcium binding proteins.
S100A4 as a prognostic factor
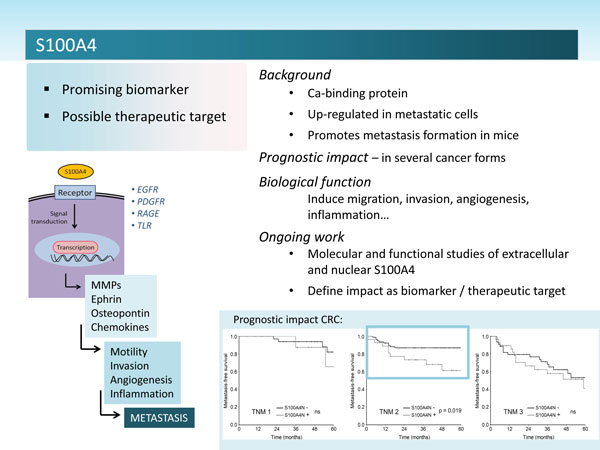
A number of reports have demonstrated that S100A4 expression could be used as a prognostic factor in a variety of different cancer types, eg.: colon cancer, breast cancer and melanoma.
S100A4 in invasion and metastasis
The biological function and molecular mechanisms by which S100A4 exerts its putative metastasis-promoting effects is largely unknown, and the protein is most likely involved in several aspects of tumor progression. One possible explanation for the multi-functionality is the fact that the protein is localized in different sub cellular compartments and probably has different binding partners in the cytoplasm, the nucleus and in the extracellular space. In the cytoplasm S100A4 co-localizes or co-sediments with different cytoskeletal proteins (actin, non-muscle myosin, and non-muscle tropomyosin), implying a possible role in cell motility and invasion. The protein has a wide variety of extracellular effects, and data indicate that release of S100A4 into the tumor microenvironment is a crucial factor in the metastatic process.
Effects of Extracellular S100A4
It has been demonstrated that in a subset of human cancer cell lines, extracellular S100A4 induce activation of the transcription factor NFkB through the classical NFkB pathway. Reports also indicate the possibility that S100A4 in the microenvironment can, as other S100 proteins, act as an Alarmin or DAMP molecule through activation of Toll-like receptors or RAGE. Furthermore, extracellular S100A4 has been shown to enhance the effect of EGFR ligands, and it has been postulated that EGFR might be a receptor for S100A4. Extracellular S100A4 induced genes include, but are not restricted to; osteopontin, uPAR, a variety of cytokines, and matrix metalloproteinases (MMPs).
S100A4 and proteases
Central metastatic properties of cancer cells imply the acquirement of invasive properties to be able to degrade and invade into surrounding mesenchyme. Previous research by us and others, have demonstrated an association between the expression of S100A4, activity of matrix metalloproteinases (MMPs) and cellular metastatic capacity. Furthermore, this regulation was suggested to involve NFκB and recently we demonstrated that downregulation of S100A4 decrease expression of the cysteine protease legumain.