Functional precision oncology
- Our team is on a mission to personalize treatment for patients with metastatic colorectal cancer (CRC). We utilize functional precision oncology, which focuses on understanding how individual live tumor cells respond to specific drugs.
- In preclinical studies, we use cell lines, organoid models, and mice to study tumor development and mechanisms by which tumors become resistant to drugs.
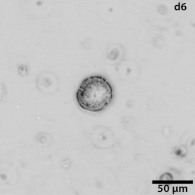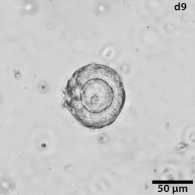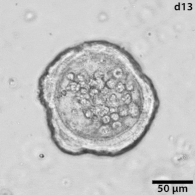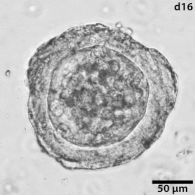 |
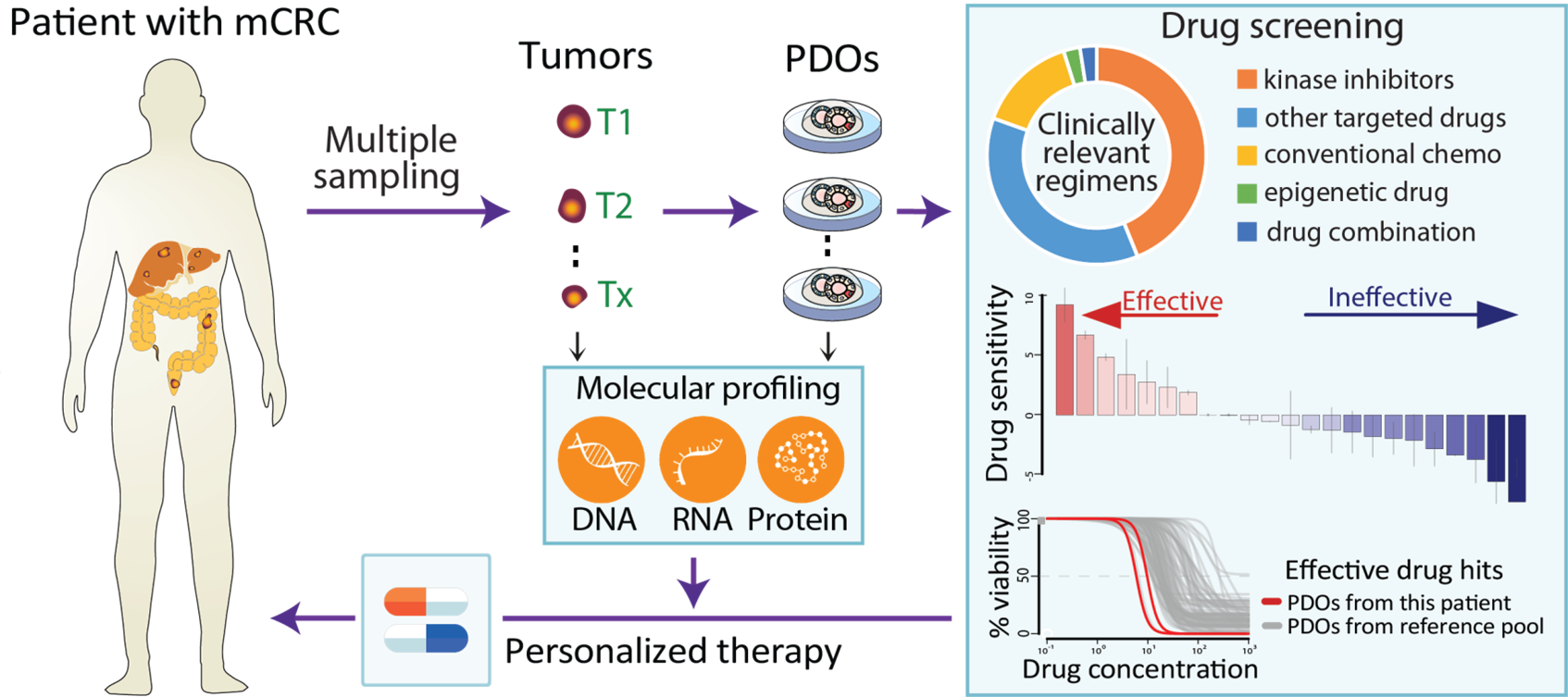
Growing tumors avatars: We establish living models of each patient's cancer by growing "mini-tumors" in the lab called patient-derived tumor organoids (PDOs). These organoids closely replicate the original tumor, allowing us to study its behavior and response to potential treatments. The living biobank of PDOs serves as a cornerstone of our functional approach to study the disease of individual patients and patient subgroups. |
Matching the right treatment: Our ultimate goal is to match each patient with the therapies most likely to benefit them based on the results of functional assays with their PDOs. By understanding how tumors evolve and adapt to treatment pressures, we can develop strategies to exploit these vulnerabilities for therapeutic benefit. This personalized approach can potentially improve treatment outcomes and reduce side effects.
Teamwork for clinical implementation: Our close collaboration with genetics, bioinformatics, pathology, surgery, and oncology experts bridges the gap between research and real-world applications. Anita Sveen's computational oncology project group closely collaborates with our team to generate and analyze multi-omics data.
Preclinical studies
- Inhibition of Poly (ADP-ribose) polymerase (PARP) in CRC. Based on our initial study of CRC cell lines (published in EBioMedicine), and the response to PARP inhibitors in a few patients in the ongoing EVIDENT trial (NCT01237067), we are now following up these initial results by investigating the mechanisms of sensitivity and resistance to PARP inhibition in individual- and subgroups of patients with metastatic CRC.
- Understand response to BRAF/EGFR inhibition in CRC. While many patients with BRAF V600E mutations benefit from a combination of BRAF and EGFR inhibitor drugs, unfortunately, only a third of them experience benefit and usually not long-lasting. We are addressing this issue by exploring mechanisms of acquired resistance, as well as molecular markers that predict response.
- E3 ubiquitin ligases influence drug activities. E3 ubiquitin ligases are enzymes that play a crucial role in regulating the levels of proteins inside cells. They work by attaching ubiquitin to other proteins, which marks them for destruction. This process is essential for many cellular functions. We are investigating how some selected E3 ubiquitin ligases affect drug sensitivity and resistance in CRC preclinical models.
For the preclinical studies, we closely collaborate with the Cell Signaling project group led by Edward Leithe.

Selected publications last 5 years:
Molecular prognostic factors for liver transplantation of unresectable metastatic colorectal cancer.
Moosavi SH, Kryeziu K, Eilertsen IA, Nunes L, Hektoen M, Niederdorfer B, Reims HM, Syversveen T, Grut H, Dueland S, Line PD, Lothe RA, Sveen A. Br J Surg. 2025 Mar 28;112(4)
Co-occurring mutations identify prognostic subgroups of microsatellite stable colorectal cancer.
Nunes L, Stenersen JM, Kryeziu K, Sjöblom T, Glimelius B, Lothe RA, Sveen A.Mol Cancer.2024 Nov 25;23(1):264
A novel EGFR inhibitor acts as potent tool for hypoxia-activated prodrug systems and exerts strong synergistic activity with VEGFR inhibition in vitro and in vivo.
Caban M, Koblmueller B, Groza D, Schueffl HH, Terenzi A, Tolios A, Mohr T, Mathuber M, Kryeziu K, Jaunecker C, Pirker C, Keppler BK, Berger W, Kowol CR, Heffeter P. Cancer Lett. 2023 Jul 1;565:216237
Multiplex immunohistochemistry of metastatic colorectal cancer and ex vivo tumor avatars.
Kryeziu K, Bergsland CH, Guren TK, Sveen A, Lothe RA. Biochim Biophys Acta Rev Cancer. 2022 Jan;1877(1):188682.
Increased sensitivity to SMAC mimetic LCL161 identified by longitudinal ex vivo pharmacogenomics of recurrent, KRAS mutated rectal cancer liver metastases.
Kryeziu K, Moosavi SH, Bergsland CH, Guren MG, Eide PW, Totland MZ, Lassen K, Abildgaard A, Nesbakken A, Sveen A, Lothe RA.J Transl Med. 2021 Sep 8;19(1):384
Molecular correlates of sensitivity to PARP inhibition beyond homologous recombination deficiency in pre-clinical models of colorectal cancer point to wild-type TP53 activity.
Smeby J, Kryeziu K, Berg KCG, Eilertsen IA, Eide PW, Johannessen B, Guren MG, Nesbakken A, Bruun J, Lothe RA, Sveen A.EBioMedicine. 2020 Sep;59:102923
Patient-Derived Organoids from Multiple Colorectal Cancer Liver Metastases Reveal Moderate Intra-patient Pharmacotranscriptomic Heterogeneity.
Bruun J, Kryeziu K, Eide PW, Moosavi SH, Eilertsen IA, Langerud J, Røsok B, Totland MZ, Brunsell TH, Pellinen T, Saarela J, Bergsland CH, Palmer HG, Brudvik KW, Guren T, Dienstmann R, Guren MG, Nesbakken A, Bjørnbeth BA, Sveen A, Lothe RA.Clin Cancer Res. 2020 Aug 1;26(15):4107-4119
