MetAction – Actionable targets in cancer metastasis
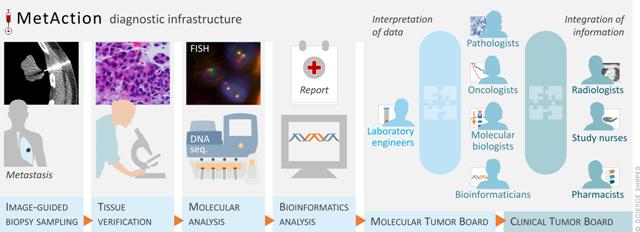
MetAction was a project funded through the Research Council of Norway (RCN), program for “Publicly funded clinical studies”, and conducted the first clinical trial in Norway where treatment decisions were based on targeted NGS data. The first part of the study (25 patients) was used to establish the infrastructure needed to be able to offer treatment based on genomic examinations; this included image-guided biopsy sampling, pathological examination, NGS sequencing, bioinformatics pipelines for variant calling and interpretation, molecular- and clinical tumor boards for discussion of molecular data and treatment alternatives. In this first part of the study, we were able only to offer single drug treatment and the samples were screened for mutation in relatively few genes (n=50), and no patients were found eligible for treatment. In the second part of the study, a more comprehensive gene panel was used, consisting of 136 genes, and combination treatment could be offered. Actionable targets were detected in 13/26 patients (15%) and long-term effects were observed in two patients.
The MetAction study demonstrated the feasibility og using targeted NGS to inform on rationale treatment for patients with end-stage metastatic disease. Furthermore, the project established and piloted the diagnostic pipeline and multidisciplinary tumor boards that has been further implemented in the national PCM study IMPRESS.