Clinical studies in breast cancer
NeoAva – Neoadjuvant Avastin in locally advanced breast cancer
PI: Olav Engebråten

Senior scientist Mads Haugen (PhD) has used machine learning principles to identify a nine-protein signature, the ViRP score, capable to identify patients who benefit from anti-angiogenic treatment with bevacizumab. The protein score is currently being validated in the NAPEER clinical study.
I-BCT -Improved Breast Cancer Therapy in the neoadjuvant and metastatic setting:
PI: Olav Engebråten
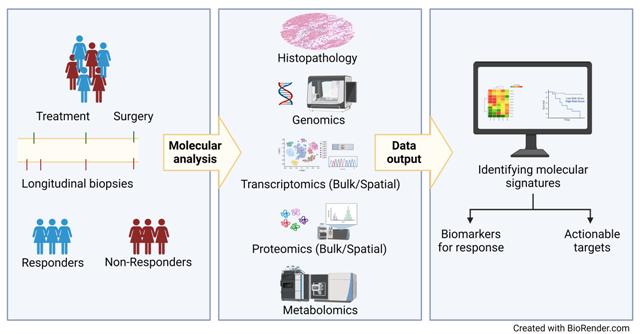
I-BCT1 is a randomized, noeadjuvant, phase II clinical study that test the hypothesis of offering additional DNA targeted chemotherapy (carboplantin) to breast cancer patients with an aggressive phenotype (inclusion ended). Patient samples (tumor and blood) were collected before start- and during treatment, and at surgery. A comprehensive translational program including bulk-, single cell- and spatial multi-omics analyses are in progress.
NAPEER - NeoAdjuvant PErsonalized therapy for Estrogen Receptor positive breast cancer
PI: Olav Engebråten
NAPEER is a randomized, neoadjuvant, phase-II study in hormone receptor positive breast cancer. In high-risk patients, chemotherapy starts immediately, while low-risk patients receive neoadjuvant endocrine therapy, randomized to treatment with or without the AKT-inhibitor capivasertib. After 15 days, the endocrine treatment response is assessed by Ki67-expression. Responding patients will continue to receive endocrine/capivasertib treatment, while non-responding patients will be switched to treatment with chemotherapy and randomized to also receive bevacizumab if positive ViRP score. Patients without a positive ViRP signature will be treated with standard chemotherapy.
Tumor biopsies and blood samples are collected longitudinally (before, during, after treatment) and will be subjected to a comprehensive translational program searching for biomarkers by bulk-, single cell- and spatial multi-omics analyses.