Therapy prediction in lung cancer

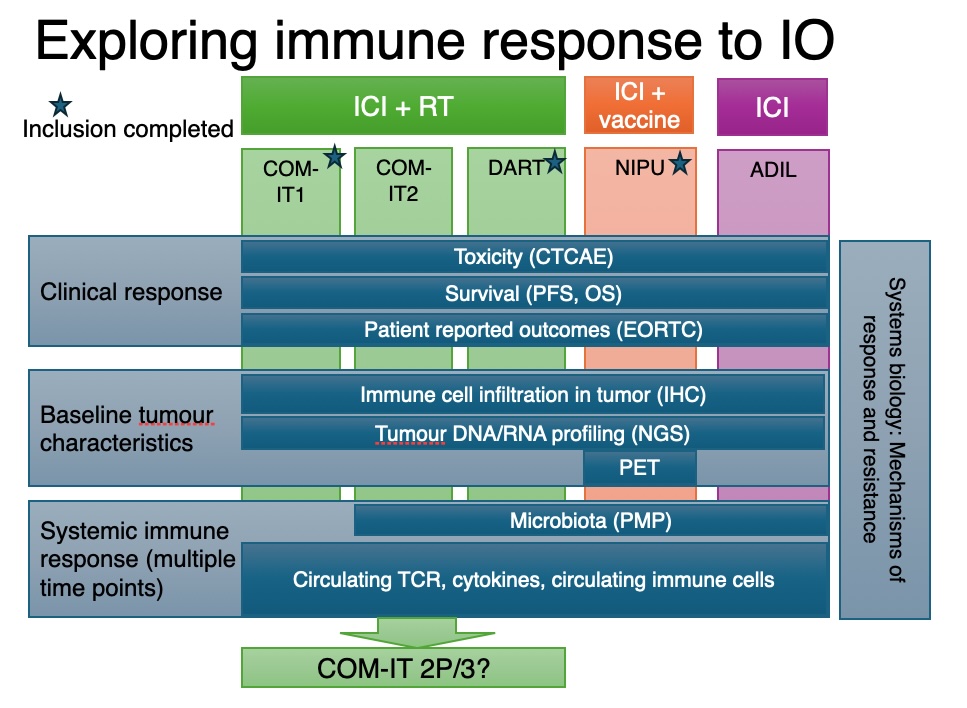
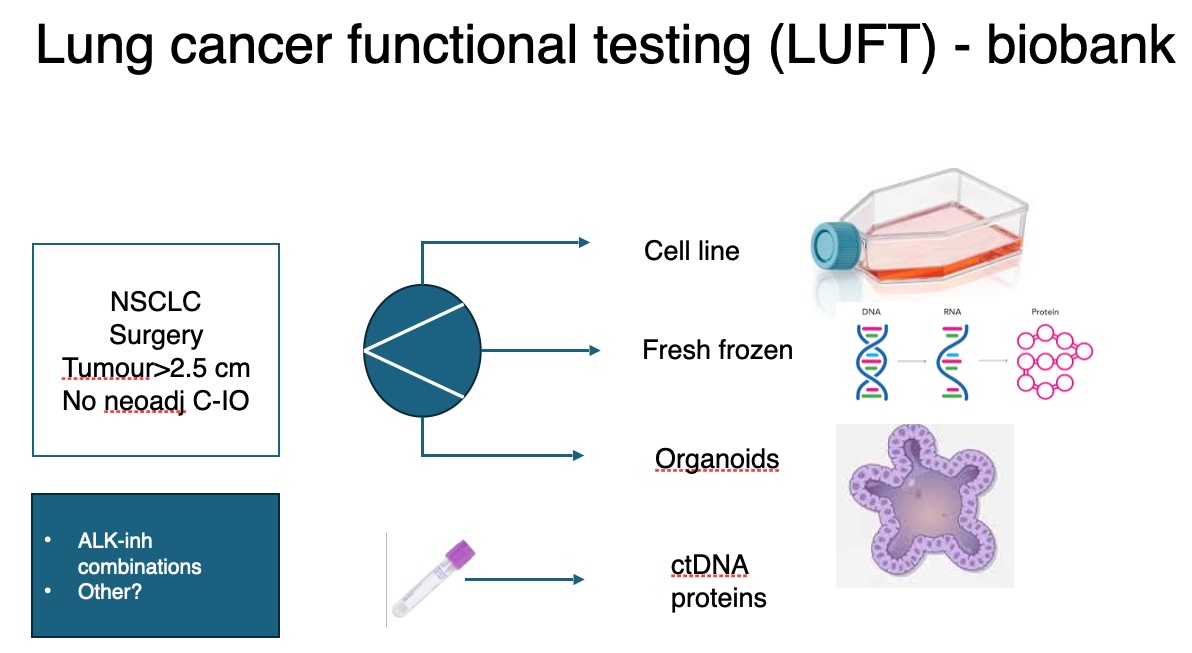
Lung cancer is the leading cause of cancer-related death. Treatment efficacy is currently limited by lack of robust predictive biomarkers to select the best treatment for an individual patient, therapy resistance and low availability of targeted therapies. To identify new predictive biomarkers and achieve better stratification of patients, we collect biological material and imaging data. Integrated analysis of profiling of primary tumour, gut microbiome, circulating markers and imaging markers are used to characterise patients and tumour diseases. The new knowledge and procedures will be used to enhance treatment and design new clinical trials.
The project group has tight collaboration with the lung cancer research group at the Dept. of Oncology.