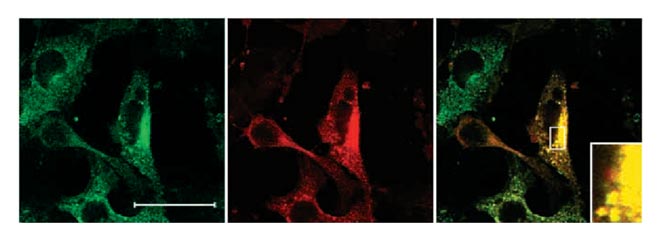
Characterisation of the intracellular routing of endocytosed FGF1.
After FGF1 is bound to its specific receptor, it is endocytosed and transported to the juxtanuclear recycling compartment. FGF1 bound to surface heparans is also endocytosed, but it is not transported to the recycling compartment, but appears to be routed to the lysosomes instead. We have made a number of mutations and deletions in the cytoplasmic part of the receptor in attempts to identify the signal determining the routing to the recycling compartment. We have found that mutations of a putative caveolin binding domain in the receptor prevents the transport to the recycling compartment. On the other hand, a receptor lacking the whole kinase domain including the putative caveolin binding domain is able to direct the growth factor to the recycling compartment provided that the remaining part of the receptor is retained. This part of the receptor is also sufficient to facilitate translocation of the growth factor to the cytosol.
We are now studying a series of mutations in the cytoplasmic part of the receptor for these two abilities. We hope the results will tell us if transport to the recycling compartment is a prerequisite for translocation of the growth factor to the cytosol and nucleus.
Endocytosis of aFGF bound to its specific receptors occurs only partly from clathrin coated pits. Thus inhibition of clathrin dependent endocytosis by acidification of the cytosol reduces the uptake only and to different extents in different cell lines. In COS cells it is almost not reduced at all, whereas in HeLa cells it is reduced to the half. In HeLa cells similar results were obtained in cells expressing inactive dynamin which also inhibits endocytosis from coated pits. We are now studying if FGF receptors can be used to characterise further the mechanism of uncoated endocytosis.
It has been claimed in the literature that FGF receptors are present in the nucleus. The mechanism for transport of the receptor to this location (if it occurs) is unclear. By immunostaining with anti-receptor 4 we have not been able to demonstrate convincingly that the receptor is in the nucleus of transfected cells. However, we have found accumulation of receptor in the perinuclear region, some of which could be present in the inner nuclear membrane. Thus it is not removed by treatment of the nuclei with Triton X-100.