Centre for Advanced Cell and Gene therapy launched

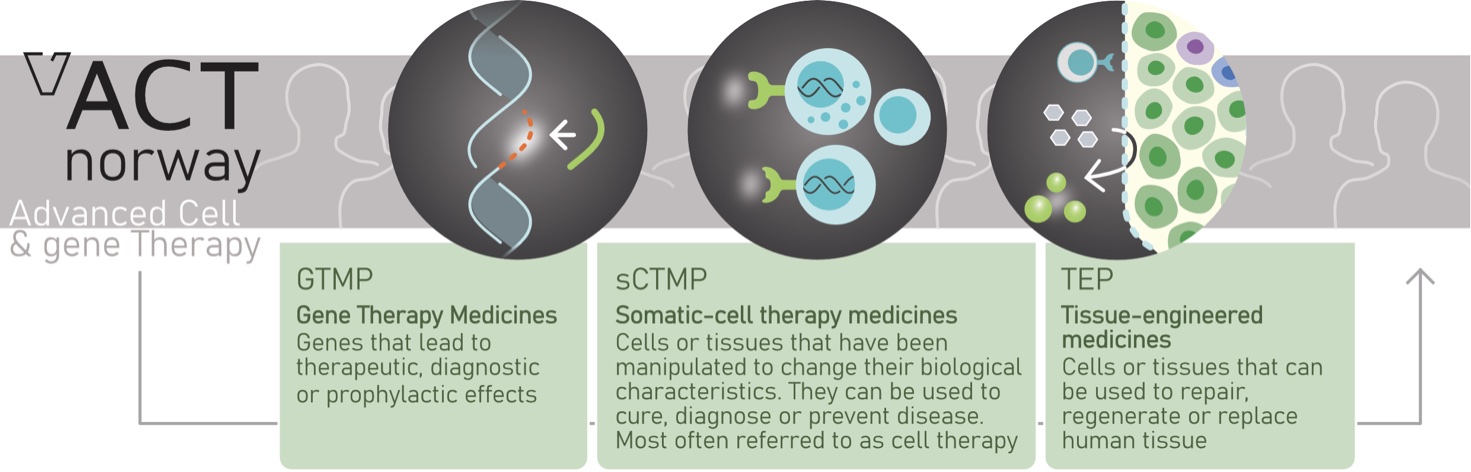
Cell and gene therapy form two of the most dynamic research areas world-wide and provide fundamentally new therapies for diseases without available treatment. Advanced therapy medicinal products (ATMPs) typically targets the underlying biology of the disease rather than the symptoms and therefore offer the possibility of cure. The development of new cell and gene therapies is spearheaded by the unprecedented clinical success of cancer immunotherapy and chimeric antigen receptor (CAR)-T cell therapy for B cell malignancies. In parallel, new advances in stem cell biology, including the possibility for differentiation of mesenchymal stem cells (MSCs), and of induced pluripotent stem cells (iPSC) reprogrammed from somatic cells, open up new possibilities to regenerate cells and tissues for the treatment of chronic diseases such as diabetes as well as various organ failures, including liver diseases. However, clinical-grade cell engineering represents a key bottleneck for the development of new ATMPs.
To address this bottleneck, a donor consortium consisting of Svanhild and Arne Must´s Foundation for Medical Research (lead donor), RADFORSK oncology research fund and the Norwegian Cancer Society, has committed 50 MNOK to form a Centre for Advanced Cell and Gene therapy (ACT Centre) located in clean room facilities at the OUH. The investment is dedicated to actions that strengthen the competence in cell differentiation, manipulation and genomic editing under full-scale good manufacturing practices (GMP).
Karl-Johan Malmberg, Scientific Director, ACT Centre:
“With the launch of the ACT centre, we outline a path to expand and restructure existing pre-GMP and GMP facilities into a common national infrastructure for ATMP manufacturing to make advanced therapies available to Norwegian patients, and to create a fundament for innovation and industrial collaborations.“
|
Goals: |
|
The Primary goal of the ACT centre is to facilitate the development of novel advanced therapy medicinal products (ATMP) and make novel therapies available to Norwegian patients.
|
Links:
From Norwegian sites:
NRK (Norwegian Broadcasting Corporation):
Report, Søndagsrevyen, May 2nd 2021:
Genbehandling mot kreft
News article:
Ble frisk fra uhelbredelig kreft på tre måneder
Kreftforeningen (Norwegian Cancer Society):
Revolusjonerende satsing
Oslo Universitetssykehus:
Nytt senter gir håp om nye behandlingsmetoder til flere pasientgrupper
Centre for Advanced Cell Therapy (ACT) home page
OUH Strategic Research Area for Cell Therapy - STRATCELL
Section for Cell Therapy, Dept. of Oncology, OUH
Dept. of of Cancer Immunology, ICR, OUH
Institute for Cancer Research (ICR), OUH
ICR Annual Report, page 17 (on ACT)
