Validation study of the LMNA-risk VTA calculator
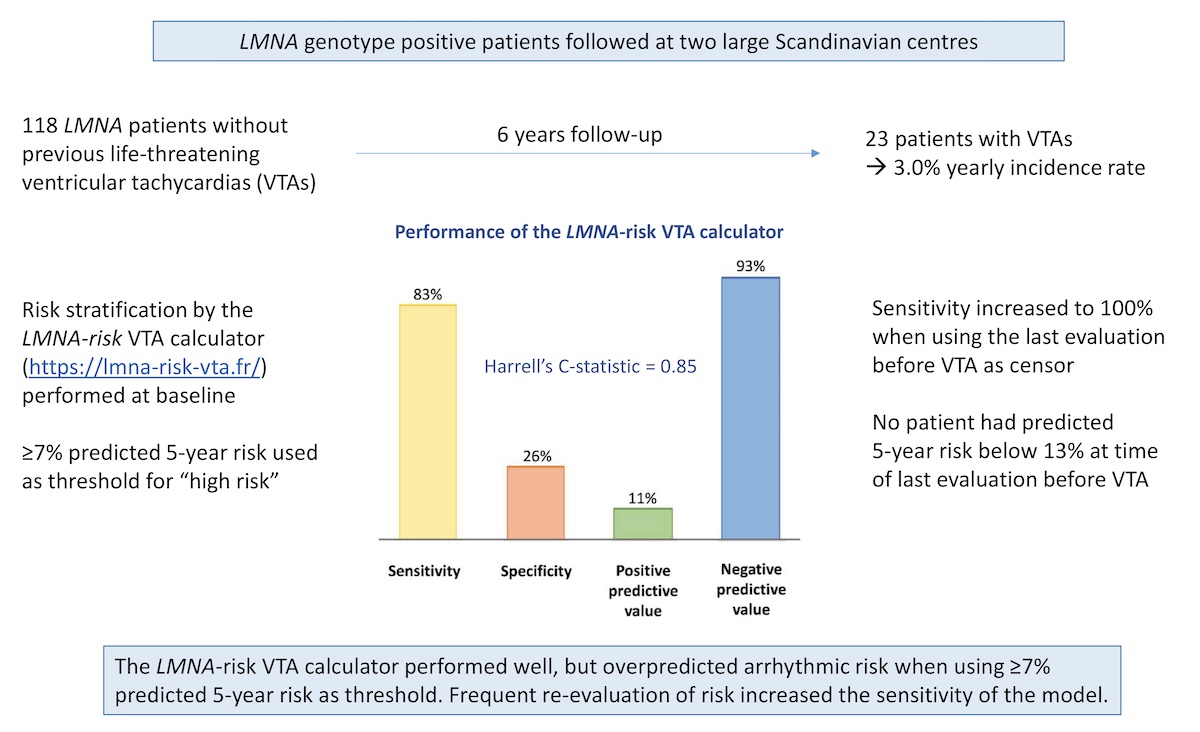
PhD-fellow Christine Rootwelt-Norberg, professor Kristina Haugaa and co-authors have published a paper validating the existing LMNA-risk VTA calculator for prediction of malignant arrhythmias in patients with cardiac laminopathies. The study was a multicentre collaboration with Rigshospitalet University Hospital in Copenhagen, including 118 LMNA genotype positive patients followed for 6 years. Twenty-three (19%) of patients experienced first-time life-threatening ventricular arrhythmias during follow-up. The previously published LMNA-risk calculator (https://lmna-risk-vta.fr/) showed high sensitivity for detecting forthcoming ventricular arrhythmias, but low specificity, and overpredicted arrhythmic risk particularly in male patients.
Cardiac laminopathies are highly malignant forms of familial dilated cardiomyopathy (DCM), caused by deleterious variants in the LMNA gene.
A large proportion of LMNA genotype-positive patients receive a primary prevention implantable cardioverter-defibrillator (ICD) to protect against life-threatening ventricular tachyarrhythmias (VTAs) and sudden death. Several previous studies have reported predictors of VTAs in LMNA genotype-positive patients including non-sustained ventricular tachycardia (NSVT), atrioventricular (AV) block, left ventricular ejection fraction (LVEF) <45%, male sex, and non-missense LMNA variants. A risk calculator for predicting VTA in laminopathies was introduced in 2019, which includes all the aforementioned predictors, and LVEF as a continuous variable. We aimed to perform an external validation of the LMNA-risk VTA calculator in a multicenter Norwegian-Danish cohort of LMNA genotype-positive patients.
We included 118 LMNA genotype-positive patients (age 37 years [IQR 27–49 years]; 39 [33%] probands; 65 [55%] women; 100 [85%] with non-missense LMNA variants). Twenty-three patients (19%) experienced VTA during 6.1 years (interquartile range 3.0–9.1 years) follow-up, resulting in 3.0% (95% confidence interval 2.0%–4.5%) yearly incidence rate. The LMNA-risk VTA calculator showed lower sensitivity, lower specificity, lower positive predictive value, lower negative predictive value, and higher proportion of ICD recipients in this validation cohort than in the original risk calculator cohort. In this external validation cohort, the LMNA-risk VTA calculator provided a 5-year sensitivity and specificity of 83% (95% CI 52%–98%) and 26% (95% CI 18%–35%), respectively, when applying the suggested ≥7% predicted 5-year risk as cutoff. The 5-year positive predictive value was 11% (95% CI 6%–20%) and the negative predictive value was 93% (95% CI 78%–99%).
This study demonstrated that the LMNA-risk VTA calculator can provide valuable guidance in clinical practice when used frequently for reevaluation of patient risk. However, the previously proposed cutoff value of ≥7% predicted 5-year risk for primary prevention ICD may result in premature implantation of devices. In particular, male risk may be overestimated by the calculator, and classification of patients as high risk on the basis of male sex and non-missense genetic variants alone may not be applicable in all populations. We suggest that male sex and a non-missense variant alone should not lead to implantation of a primary prevention ICD. We highlight the importance of close follow-up in these patients, as risk prediction was most accurate when using the most recent patient data.
Timing of cardioverter-defibrillator implantation in patients with cardiac laminopathies—External validation of the LMNA-risk ventricular tachyarrhythmia calculator - ScienceDirect
Heart Rhythm. 2022 Dec 6;S1547-5271(22)02686-8.
Christine Rootwelt-Norberg, Alex Hørby Christensen, Eystein T Skjølsvik, Monica Chivulescu, Christoffer R Vissing, Henning Bundgaard, Eivind W Aabel, Martin P Bogsrud, Nina E Hasselberg, Øyvind H Lie, Kristina H Haugaa
PMID: 36494026
DOI: 10.1016/j.hrthm.2022.11.024
Shared under Creative Commons (CC BY 4.0) license.
Relevant clinical trials
This is a randomized, double-blind, placebo-controlled study in patients with dilated cardiomyopathy (DCM) due to a mutation of the gene encoding the lamin A/C protein (LMNA). The study will further evaluate a dose level of study drug (ARRY-371797) that has shown preliminary efficacy and safety in this patient population. After the primary analysis has been performed, eligible patients may receive open-label treatment with ARRY-371797.
