Stem cells from the adult human brain
Background
A central dogma in neuroscience has been that the mature brain is unable to produce new neurons. Towards the end of the 20th century, studies in birds and rodents came to question this doctrine as new markers for labeling neurons combined with techniques for identifying cells that had been born in adult life, suggested that new neurons sometimes may develop later in life in some species.
At the turn of the century, these findings were to some degree extended to the human brain, as a few research groups had been able to cultivate immature cells from the human ventricular wall and hippocampus. It was still not known, however, whether it would be possible to differentiate these cells into functional neurons, i.e. cells with typical neuronal action potentials with the ability to communicate via synapses.
Results
The putative existence of an adult human brain stem cell type with the ability to proliferate and differentiate into mature neurons created huge interest as one may now envisage treatment of neurological diseases with either transplantation of stem cells that have been expanded in vitro or by mobilization of endogenous progenitor cells.
The work on developing functional neurons from cells in the human ventricular wall was started in Professor Langmoen’s laboratory at the Karolinska Institute in Stockholm by Morten Moe and Mercy Varghese. Human tissue was harvested from the wall of the lateral ventricle in temporal lobe specimens resected due to epilepsy. In keeping with earlier results from other groups they were able to expand stem cells from the ventricular wall as cell clusters (neurospheres) in vitro. Following dissociation and exposure to differentiation cues (mainly withdrawal of growth factors and addition of serum) these cells went through characteristic steps of morphological and electrophysiological development and developed into the three principal building blocks of the brain:
- Astrocytes
- Oligodendrocytes
- Neurons
|
Our group was first to demonstrate that it is possible to transform immature progenitor cells from the adult human ventricular zone into functional neurons, i.e. cells with typical neuronal action potentials and the ability to communicate through synapses (fig. 1). Essentially, our group was able to develop a small nervous system from a single human stem cell in vitro. This "nervous system", consisting of glial cells as well as a large number of neurons, communicated via synapses. These results were selected from more than 15000 abstracts for presentation at the press conference of the Society for Neuroscience three years ago and have been published in Brain, 2005. |
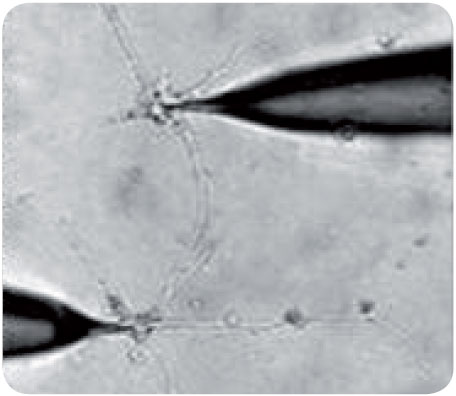 Figure 1 Dual patch-clamp recording from neighboring neuron-like cells, demonstrating synaptic communication between the cells |
An arena where adult human brain stem cells hold tremendous promise is Parkinson's disease. Parkinson's disease is characterized by loss of pigmented dopamine-secreting cells in the substantia nigra. Though transplantation of embryonic stem cell- or fetal stem cell-derived dopaminergic cells has shown promising results, the results have not been consistent. Furthermore, the ethical issues regarding the use of the aforementioned cell types, limit their clinical use. Dopaminergic cells derived from adult human brain stem cells have obvious benefits: firstly, they pose no ethical challenges and secondly, they make autologous transplantation possible. We are investigating the potential of adult human brain stem cells to develop into dopaminergic neurons. We have shown that in vitro, adult human brain stem cells develop into neurons expressing tyrosine hydroxylase (Fig. 2), a rate-limiting enzyme in dopamine synthesis. These cells have also been transplanted into mouse brain and preliminary results show that the cells survive and differentiate in vivo. The cells' phenotype is currently being investigated.
Importantly, no tumours were seen upon transplantation of these cells.
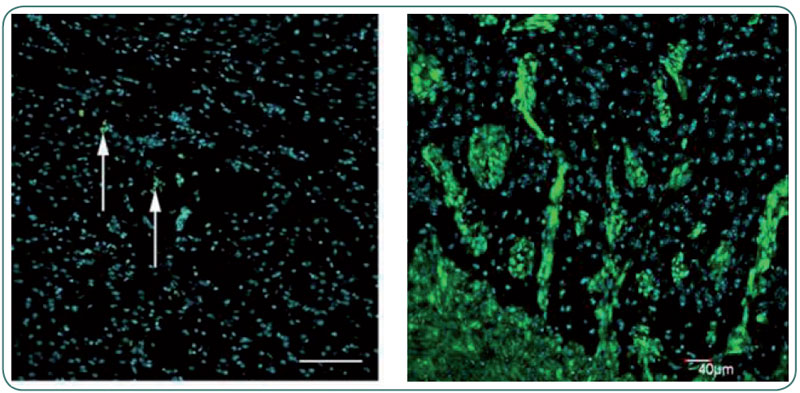 |
| Figure 2 Transplantation of normal progenitor cells from the adult human brain (left panel, green) did not result in tumor formation, whereas stem cells isolated from brain tumors (green, right panel) reproduced a highly invasive tumor in the mouse brain. Blue nuclear staining in both panels. |
For stem cells to be useful in the clinical situation, it must be demonstrable that after transplanting them to another adult brain they can survive and integrate into the recipient neuronal circuitry. Using rats with a selective lesion of the hippocampal CA1-region (a small part of cerebral gray matter), Håvard Ølstørn and Morten Moe demonstrated that stem cells from the adult human brain are not only able to survive in the rat brain, but also selectively target and migrate to the area with the lesion.
Use of specific antibodies against human nuclei (HuN) demonstrated survival of the transplanted cells and showed that the grafted cells frequently express the immature marker human nestin. Less frequently, the cells expressed the immature neuronal marker doublecortin (DCX) and the glial marker GFAP. Ølstørn and Moe further showed that by using ‘predifferentiation', i.e. "pushing" the cells in a neuronal direction prior to transplant, it was possible to significantly enhance the development of neurons following transplantation.
This study for the first time showed that stem cells from the adult human brain are able to survive and differentiate in another adult brain. The results were presented at the International Society for Stem Cell Research in Toronto 2006 and have now been published in Neurosurgery, 2007. The first part of the study secured Ølstørn the first price at the Scandinavian Neurosurgical Society's Annual Congress in 2005.
Stem cells isolated from adult human filum terminale. Mercy Varghese and coworkers have isolated neural progenitors from the adult human filum terminale (FNTPs). This terminal end of the spinal cord has been referred to as a fibrovascular tag without neurogenic potential and of no clinical significance. Similar to brain stem cells mentioned above these cells from the filum terminale generated functional neurons capable of firing action potentials. When transplanted into the adult CNS, FTNPs survived, differentiated and showed targeted migration to site of injury. These results were published in Stem Cells and Development, 2008.
